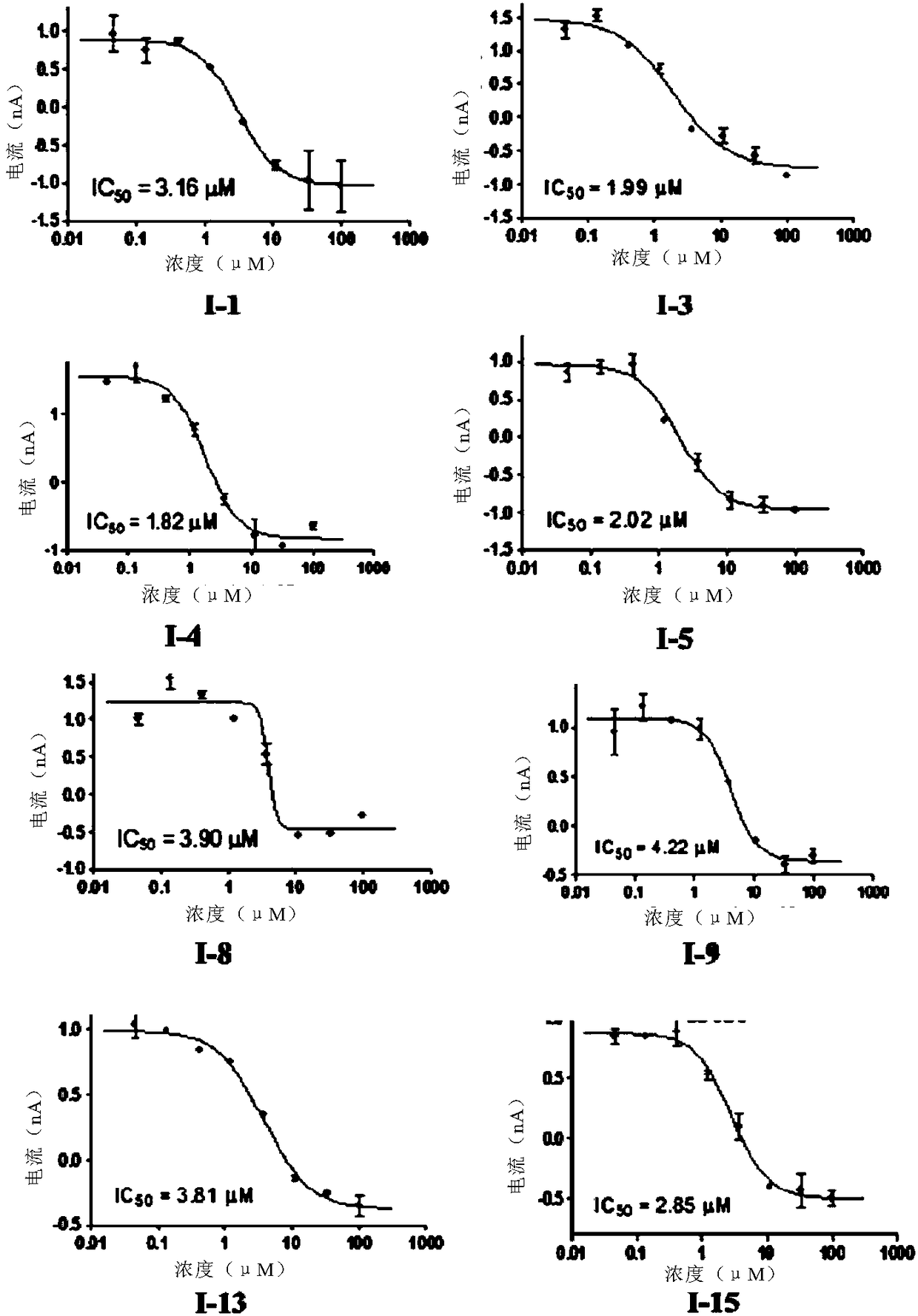
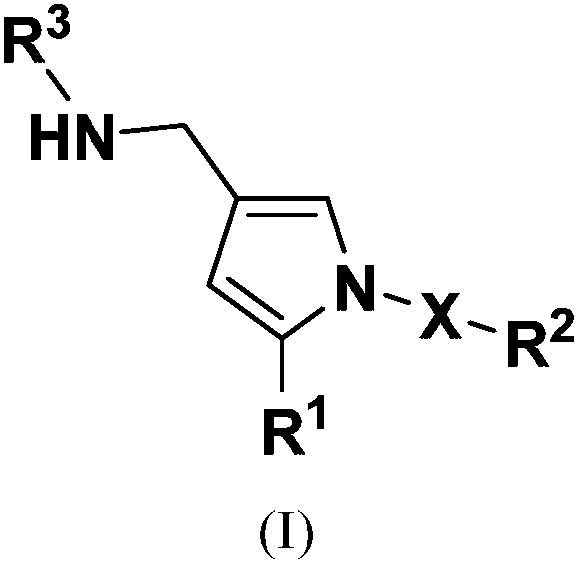
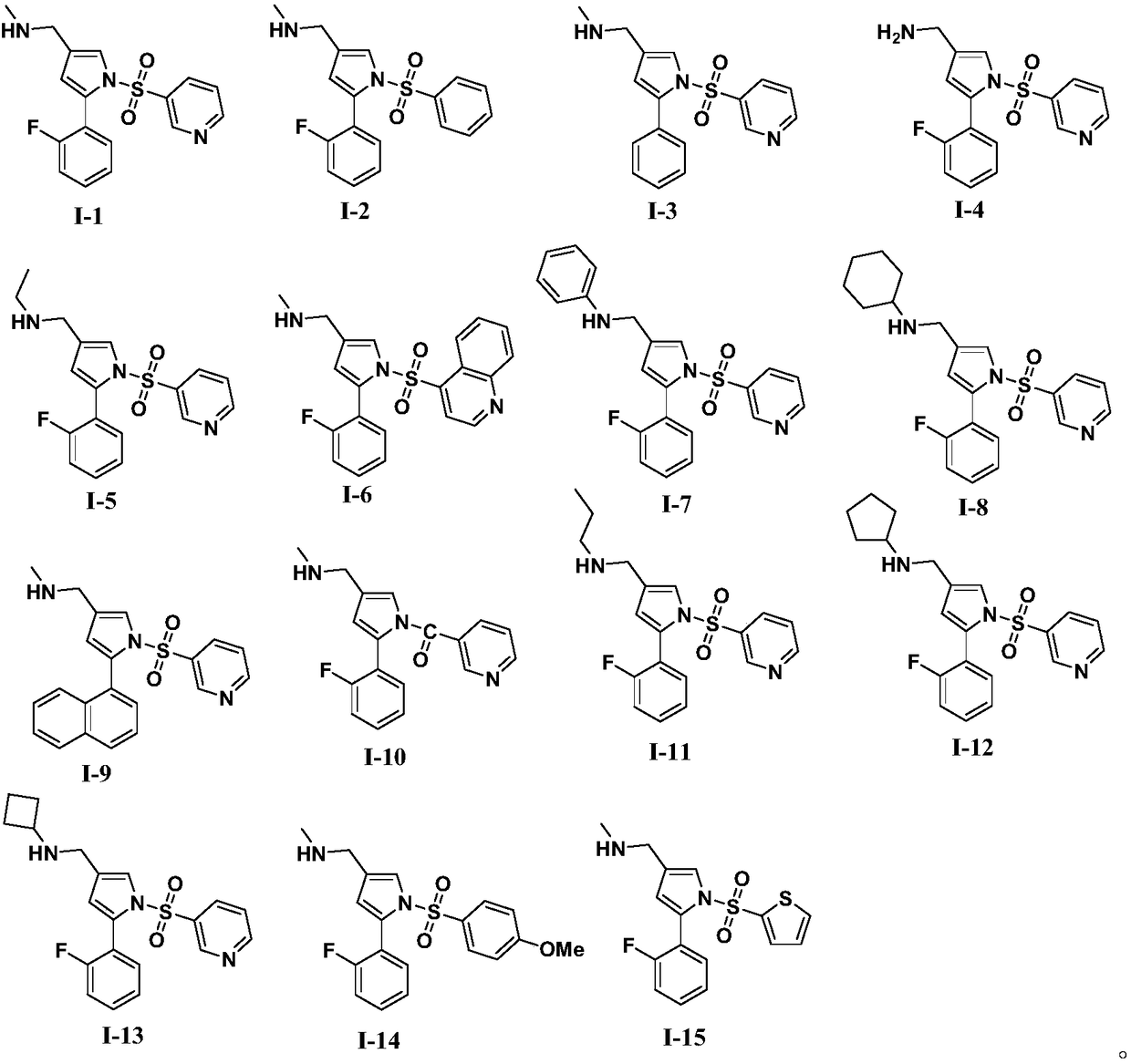
Substituted pyrrole-4-alkylamine compounds and application thereof
A technology for compounds, uses, and applications in the fields of medicinal chemistry and pharmacotherapeutics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0142] Preparation of 2-bromo-1-(2-fluorophenyl)ethan-1-one (intermediate II-1)
[0143]
[0144] Dissolve 3.5 g of 2-fluoroacetophenone in 60 ml of ethyl acetate, add 11.3 g of copper bromide, and then heat to 80° C. to react overnight. After the reaction was complete, the reaction liquid was cooled to room temperature, suction filtered, the filtrate was concentrated, and the residue was separated by silica gel column chromatography to obtain the compound of formula II-1, 5.2 g of yellow oil, with a yield of 95%.
[0145] 1 H NMR (400MHz, CDCl 3 )δ7.94(td, J=7.6,1.8Hz,1H),7.63–7.55(m,1.8Hz,1H),7.27(dd,J=11.3,3.8Hz,1H),7.18(dd,J=11.5 ,8.6Hz,1H),4.53(d,J=2.4Hz,2H).
Embodiment 2
[0147] Preparation of 2-[2-(2-fluorophenyl)-2-oxoethyl]malononitrile (intermediate III-1)
[0148]
[0149]Dissolve 5 grams of intermediate II-1 in 40 milliliters of ethanol, add 1.52 grams of malononitrile, stir until fully dissolved, slowly add 1.3 grams of potassium hydroxide in 40 milliliters of aqueous solution, dropwise for about 1 hour, and then place The reaction was stirred at room temperature for 1 hour. After the reaction was complete, the reaction solution was poured into an equal volume of water, stirred thoroughly, filtered with suction, and the filter cake was retained to obtain the compound of formula III-1, 4.2 g of a white solid compound, with a yield of 91%.
[0150] 1 H NMR (400MHz, CDCl 3 )δ8.02(td, J=7.7,1.8Hz,1H),7.71–7.60(m,1H),7.32(dd,J=15.9,8.2Hz,1H),7.23(dd,J=11.5,8.4Hz ,1H),4.39(t,J=6.6Hz,1H),3.76(dt,J=11.9,5.9Hz,2H).
Embodiment 3
[0152] Preparation of 2-chloro-5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile (intermediate IV-1)
[0153]
[0154] 2 g of intermediate III-1 were dissolved in 40 ml of hydrochloric acid in ethyl acetate, and the reaction was stirred overnight at room temperature. After the reaction was complete, the solvent was distilled off under reduced pressure to obtain the compound of formula IV-1, 2.1 g of white solid, with a yield of 96%.
[0155] 1 H NMR (400MHz, DMSO-d 6 )δ7.72(td, J=7.9,1.7Hz,1H),7.42–7.23(m,3H),6.91(d,J=2.5Hz,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com