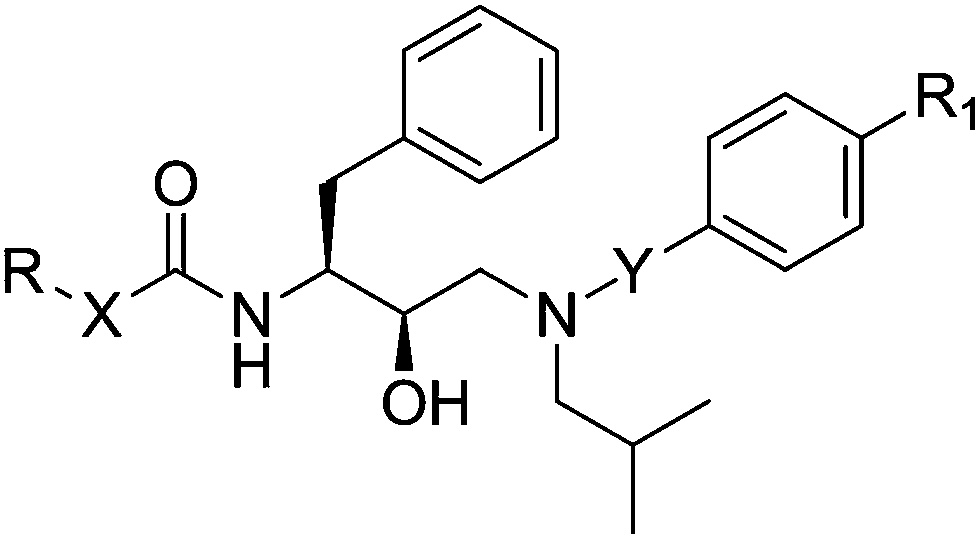
Nucleic acid base compound or medically acceptable salt thereof and preparation method and application of compound or salt thereof
A nucleic acid base and compound technology, applied in the field of nucleic acid bases, can solve the problems of HIV-1 protease activity and reverse transcriptase activity AIDS derivatives that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0084] The present invention provides the preparation method of nucleic acid base compound described in above-mentioned technical scheme, comprises the following steps:
[0085] When X in the compound of the structure shown in formula I is -CH 2 -, when R is Ra or Rb, the compound having the structure shown in Formula II-1 is condensed with the amine derivative under the action of a catalyst to obtain the compound having the structure shown in Formula I;
[0086]
[0087] In formula II-1, R is Ra or Rb, and said Ra is The Rb is Among them, R 2 is hydrogen, hydroxyl, amino or halogen; R 3 is hydrogen, hydroxy, amino, halogen or methyl; R 4 is hydrogen or methyl;
[0088] When X in the compound of the structure shown in formula I is -CH 2 -, -O-, -S- or -NH-, when R is Rc or Rd, the compound having the structure shown in formula II-2, trichloromethyl carbonate and amine derivatives are subjected to a condensation reaction under the action of a catalyst , to obtain a ...
Embodiment 1
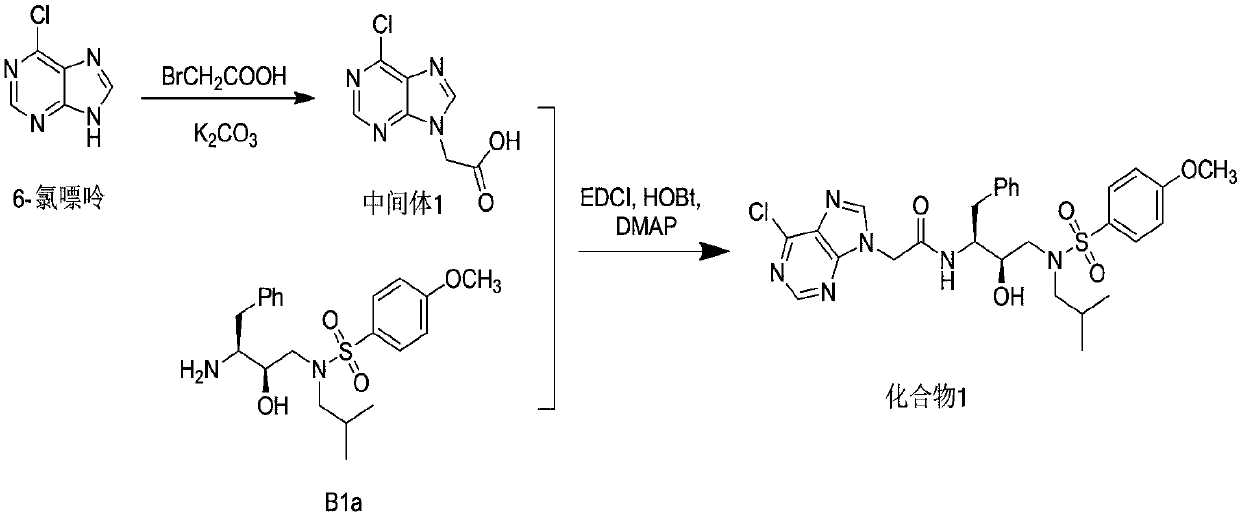
[0165] Add 6-chloropurine (0.62g, 4mmol) and anhydrous potassium carbonate (1.66g, 12mmol) into a 10mL eggplant-shaped flask, add 2mL of anhydrous solvent N,N-dimethylformamide (DMF), and The reaction was vigorously stirred at room temperature under protection for 1 h; bromoacetic acid (0.64 g, 4.6 mmol) was dissolved in 1 mL of anhydrous solvent N,N-dimethylformamide (DMF), and slowly added dropwise to the reaction solution. After the addition was complete, continue The reaction was stirred overnight at room temperature. Add 5 mL of distilled water to the reaction solution, filter the solution with diatomaceous earth, wash with water to obtain a yellow clear liquid, adjust the pH to 3.0 with 4M HCl under ice bath stirring, stir for 10 min under ice bath, stand still, suction filter, filter The cake was washed with a small amount of water and dried to obtain the target product as a yellow-green powder solid, Intermediate 1 (0.37 g, 43.4%). LC-MS of Intermediate 1 (ESI, M+H +...
Embodiment 2
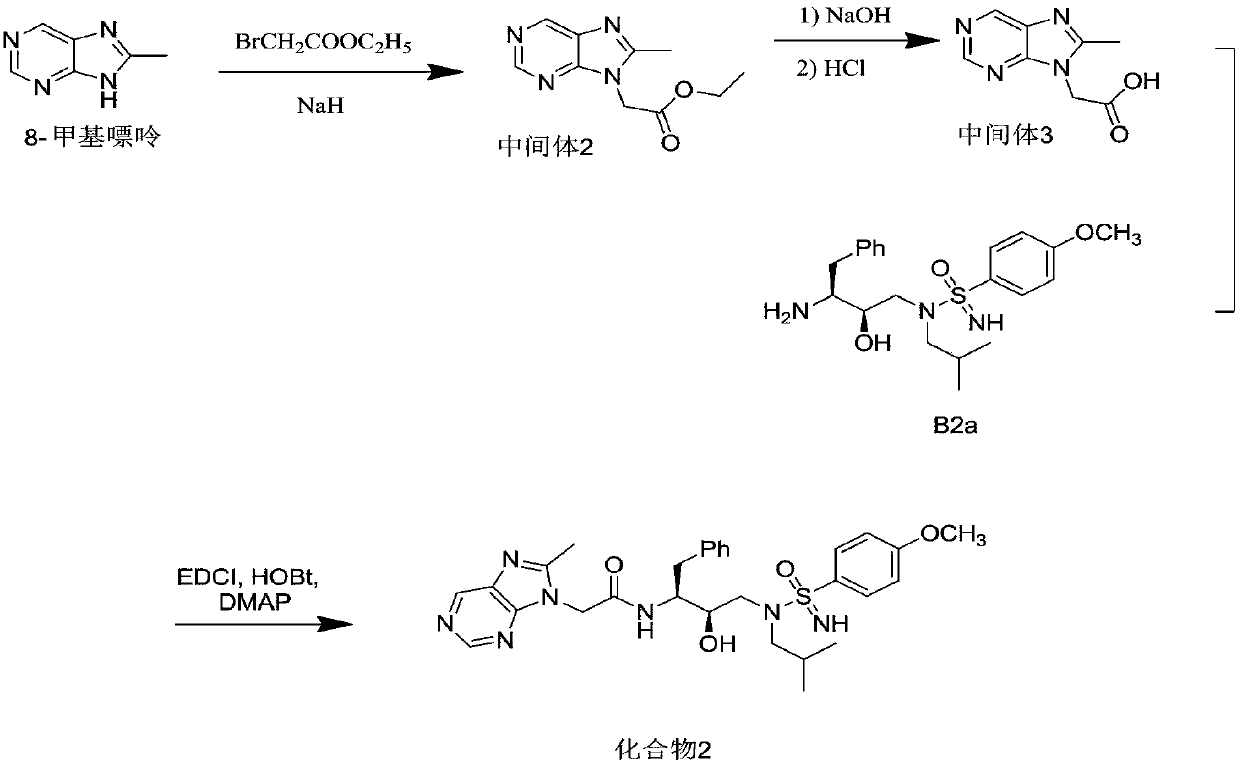
[0172] Add 8-methylpurine (1.34g, 10mmol) into a 50mL eggplant-shaped flask, add 10mL of anhydrous DMF, place in an ice bath, slowly add sodium hydride (0.44g, 11mmol) in batches, and transfer to room temperature The reaction was carried out for 1 h, ethyl bromoacetate (1.84 g, 11 mmol) was slowly added dropwise, and the reaction was continued for 1 h. After terminating the reaction, 10 mL of water was added, extracted with ethyl acetate (15×3 mL), the organic phase was dried over anhydrous sodium sulfate, and concentrated to obtain a yellow oily crude product. The crude product was purified by flash column (eluents used were ethyl acetate and hexane, volume ratio, ethyl acetate: hexane = 1:2) to obtain colorless needle crystals, intermediate 2 (1.53 g, 69.6%). LC-MS of intermediate 2 (ESI, M+H + ) m / z 221.4.
[0173] NaOH (0.65g, 16.2mmol) was dissolved in 5mL of water, added to a 100mL eggplant-shaped flask containing intermediate 2 (1.19g, 5.4mmol), stirred at room temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com