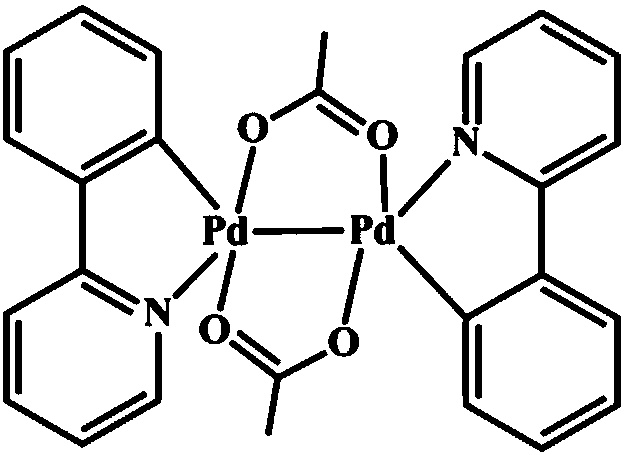
2-phenylpyridine dual-core palladium (II) complex and preparing method and application thereof
A technology of phenylpyridine and complex, applied in the field of 2-phenylpyridine binuclear palladium complex and preparation thereof, can solve the problems of side effects, large cytotoxicity, drug resistance and the like in patients, and achieve good potential medicinal value , significant in vitro antitumor activity and cytotoxicity selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Weigh 1.0mol palladium acetate and 1.0mol 2-phenylpyridine, place in a 100mL round bottom flask, add 5.0mL glacial acetic acid, that is, the mol ratio of palladium acetate, 2-phenylpyridine and glacial acetic acid is 1: 1:0.0875, mix well to obtain a mixed solution.
[0029] (2) Heating and stirring the mixed solution, allowing it to fully react at 100° C. for 24 h, then cooling to room temperature, removing the solvent, and obtaining a small amount of reddish-brown liquid.
[0030] (3) Add 3.0mL of methanol and 2.0mL of chloroform to the reddish-brown liquid and let it stand for 34 days until the yellow crystals are completely precipitated to obtain the 2-phenylpyridine dinuclear palladium (II) complex with a yield of 98.3% .
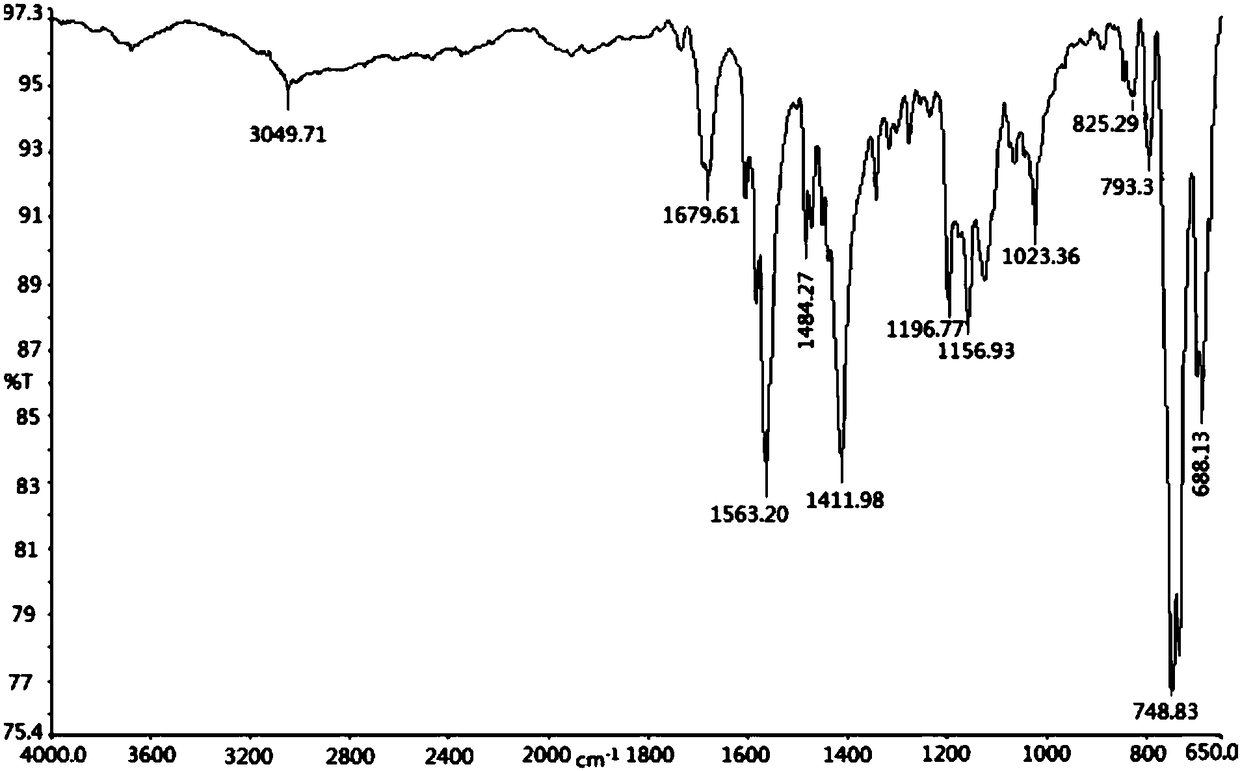
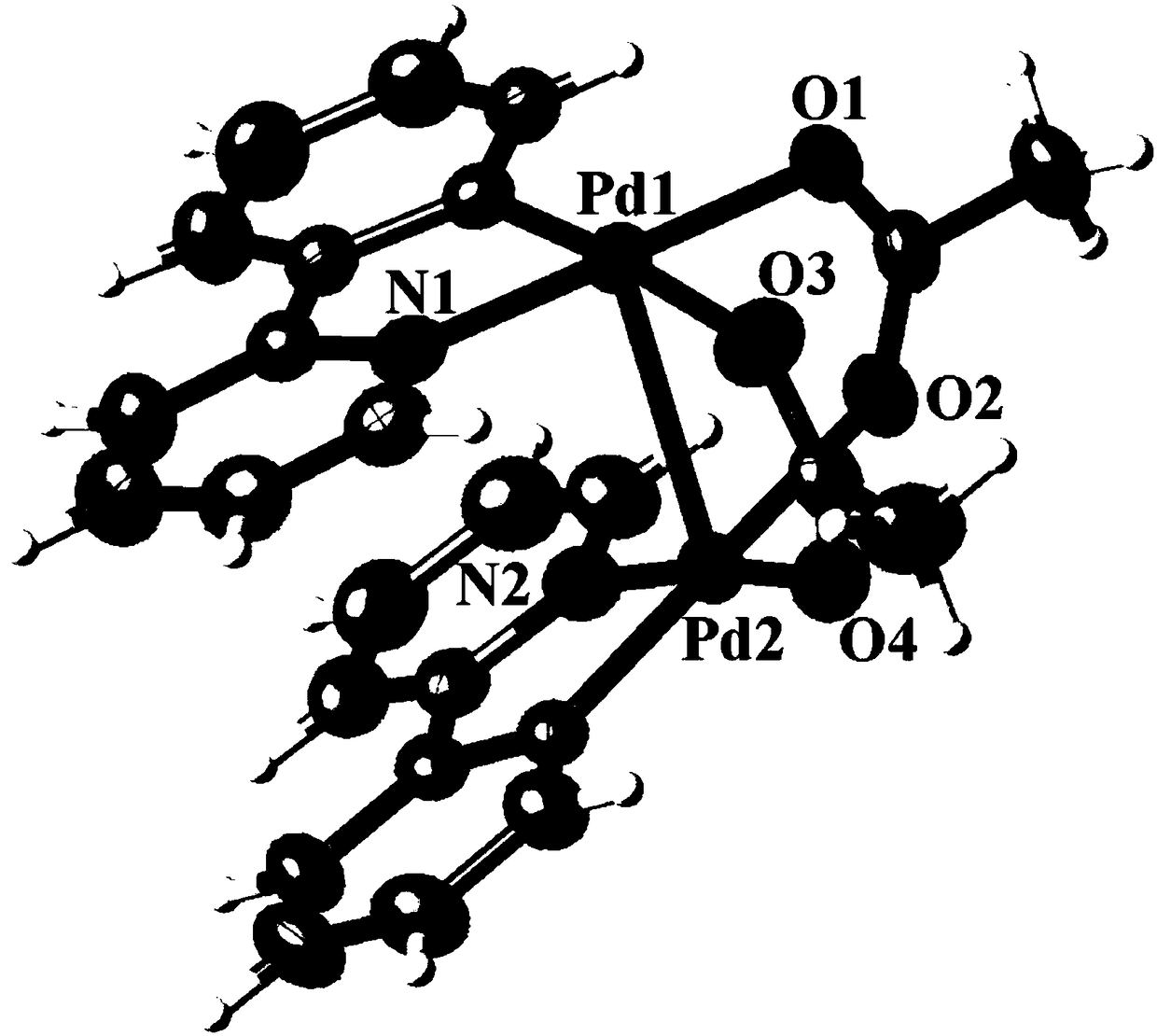
[0031] (4) The yellow crystal separated out is subjected to infrared spectroscopy (see figure 1 ), X-ray single crystal diffraction (see figure 2 ) and elemental analysis (see Table 2) determination. Infrared spectrum IR (KBr): 1680cm ...
Embodiment 2
[0035] (1) Weigh 1.0mol palladium acetate and 1.0mol 2-phenylpyridine, place in a 100mL round bottom flask, add 5.0mL glacial acetic acid, that is, the mol ratio of palladium acetate, 2-phenylpyridine and glacial acetic acid is 1: 1:0.0875, mix well to obtain a mixed solution.
[0036] (2) Heating and stirring the mixed solution, allowing it to fully react at 60° C. for 70 h, then cooling to room temperature, and removing the solvent to obtain a small amount of reddish-brown liquid.
[0037] (3) Add 92mL of ethanol and 2mL of acetone into the reddish-brown liquid and let it stand for 34 days until the yellow crystals are completely precipitated to obtain the 2-phenylpyridine dinuclear palladium(II) complex with a yield of 85.0%.
[0038] (4) The precipitated yellow crystals were subjected to the same measurement as in Example 1, and the measurement results were the same as those in Example 1. It was determined that the yellow crystals were 2-phenylpyridine binuclear palladium ...
Embodiment 3
[0040] (1) Weigh 1.0mol palladium acetate and 1.0mol 2-phenylpyridine, place in a 100mL round bottom flask, add 5.0mL glacial acetic acid, that is, the mol ratio of palladium acetate, 2-phenylpyridine and glacial acetic acid is 1: 1:0.0875, mix well to obtain a mixed solution.
[0041] (2) Heating and stirring the mixed solution, allowing it to fully react at 135° C. for 10 h, cooling to room temperature, and removing the solvent to obtain a small amount of reddish-brown liquid.
[0042] (3) Add 50 mL of ethanol and 3 mL of dichloromethane into the reddish-brown liquid, and let it stand for 34 days until the yellow crystals are completely precipitated to obtain 2-phenylpyridine binuclear palladium (II) complex with a yield of 90.0%.
[0043] (4) The precipitated yellow crystals were subjected to the same measurement as in Example 1, and the measurement results were the same as those in Example 1. It was determined that the yellow crystals were 2-phenylpyridine binuclear pallad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com