Degradable antibacterial and hemostatic material based on porous structure carrier and preparation method thereof
A technology of porous structure and hemostatic material, applied in pharmaceutical formulations, pharmaceutical sciences, absorbent pads, etc., can solve the problems of inability to achieve rapid hemostasis, adverse effects on the human body, and inability to degrade materials, achieve good synergistic effects, improve hemostatic effects, and improve Effects of fluid absorption and hemostasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
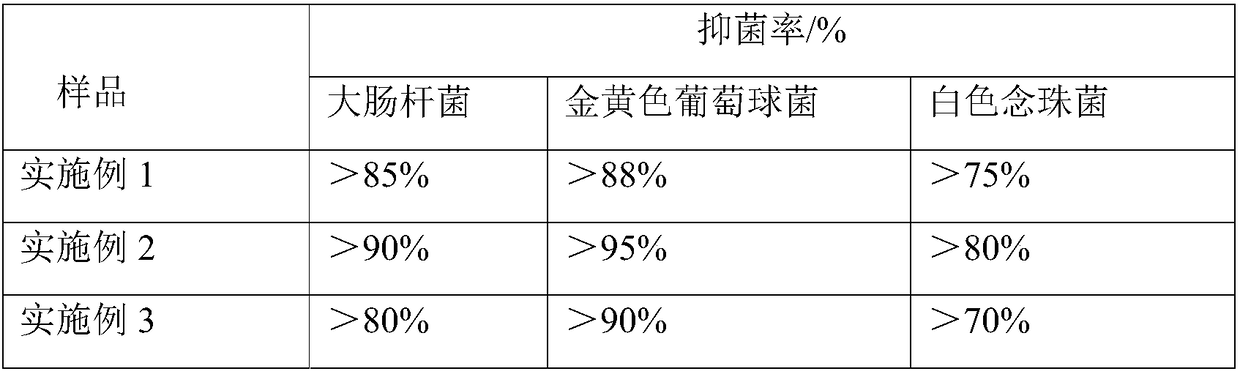
Embodiment 1
[0025] A degradable antibacterial and hemostatic material based on a porous structure carrier, in parts by weight, including 20 parts of chitosan hydrochloride, 80 parts of sodium carboxymethylcellulose, 0.5 parts of calcium-containing organic compound calcium gluconate, gram of antibacterial drug Linmycin 0.1 part.
[0026] Its preparation method comprises the following steps:
[0027] (1) Take sodium carboxymethylcellulose (degree of substitution 0.9) by weight, and slowly add sodium carboxymethylcellulose into distilled water to prepare an aqueous solution with a concentration of 2.5g / L;
[0028] (2) take chitosan hydrochloride (deacetylation degree is 70%, weight average molecular weight 280000) by weight, and slowly add in the aqueous solution that step (1) forms, and fully stir and vibrate, after adding, Continue stirring and shaking for 3 to 5 minutes to prepare a slurry composition A with a concentration of 0.5 g / L;
Embodiment 2
[0033] A degradable antibacterial and hemostatic material based on a porous structure carrier, in parts by weight, including 60 parts of chitosan hydrochloride, 40 parts of sodium carboxymethylcellulose, 1 part of calcium-containing organic compound calcium gluconate, gram of antibacterial drug Linmycin 0.5 part.
[0034] Its preparation method comprises the following steps:
[0035] (1) Take sodium carboxymethylcellulose (degree of substitution 0.9) by weight, and slowly add sodium carboxymethylcellulose into distilled water to prepare an aqueous solution with a concentration of 2.5g / L;
[0036] (2) Take chitosan hydrochloride (degree of deacetylation is 80%, weight average molecular weight 350000) by weight, and slowly add in the aqueous solution that step (1) forms, and fully stir and vibrate, after adding, Continue stirring and shaking for 3-5 minutes to prepare a slurry composition A with a concentration of 2 g / L;
[0037] (3) Freeze-drying: The prepared slurry composit...
Embodiment 3
[0041] A degradable antibacterial and hemostatic material based on a porous structure carrier, comprising 30 parts of chitosan hydrochloride, 70 parts of carboxymethyl cellulose sodium, 1.5 parts of calcium citrate, an antibacterial drug red Mycin 0.5 part.
[0042] Its preparation method comprises the following steps:
[0043] (1) Take sodium carboxymethyl cellulose (degree of substitution 1.5) by weight, and slowly add sodium carboxymethyl cellulose into distilled water to prepare an aqueous solution with a concentration of 2.5g / L;
[0044](2) Take chitosan hydrochloride (deacetylation degree is 90%, weight average molecular weight 500000) by weight, and slowly add in the aqueous solution that step (1) forms, and fully stir and vibrate, after adding, Continue stirring and shaking for 3 to 5 minutes to prepare a slurry composition A with a concentration of 5 g / L;
[0045] (3) Freeze-drying: The prepared slurry composition A with a concentration of 5 g / L was first frozen at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com