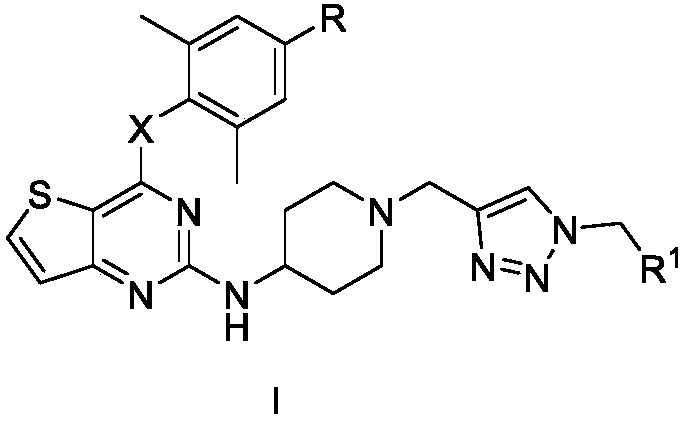
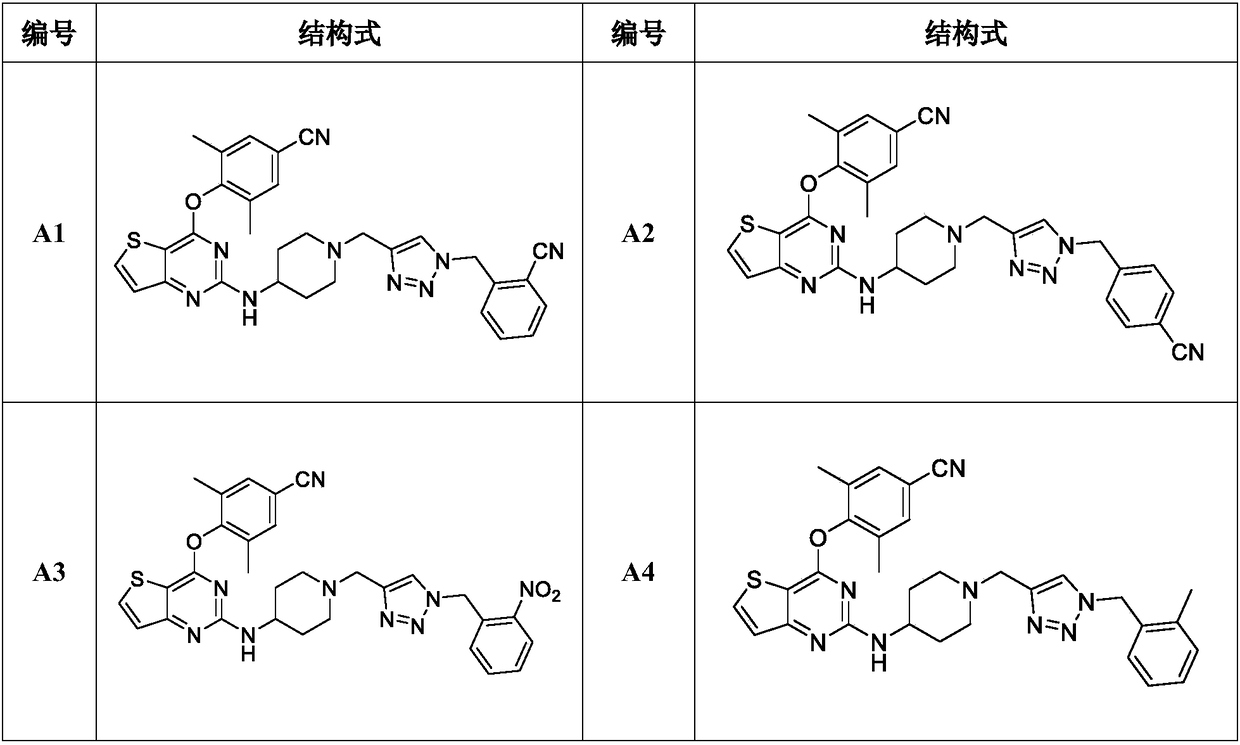
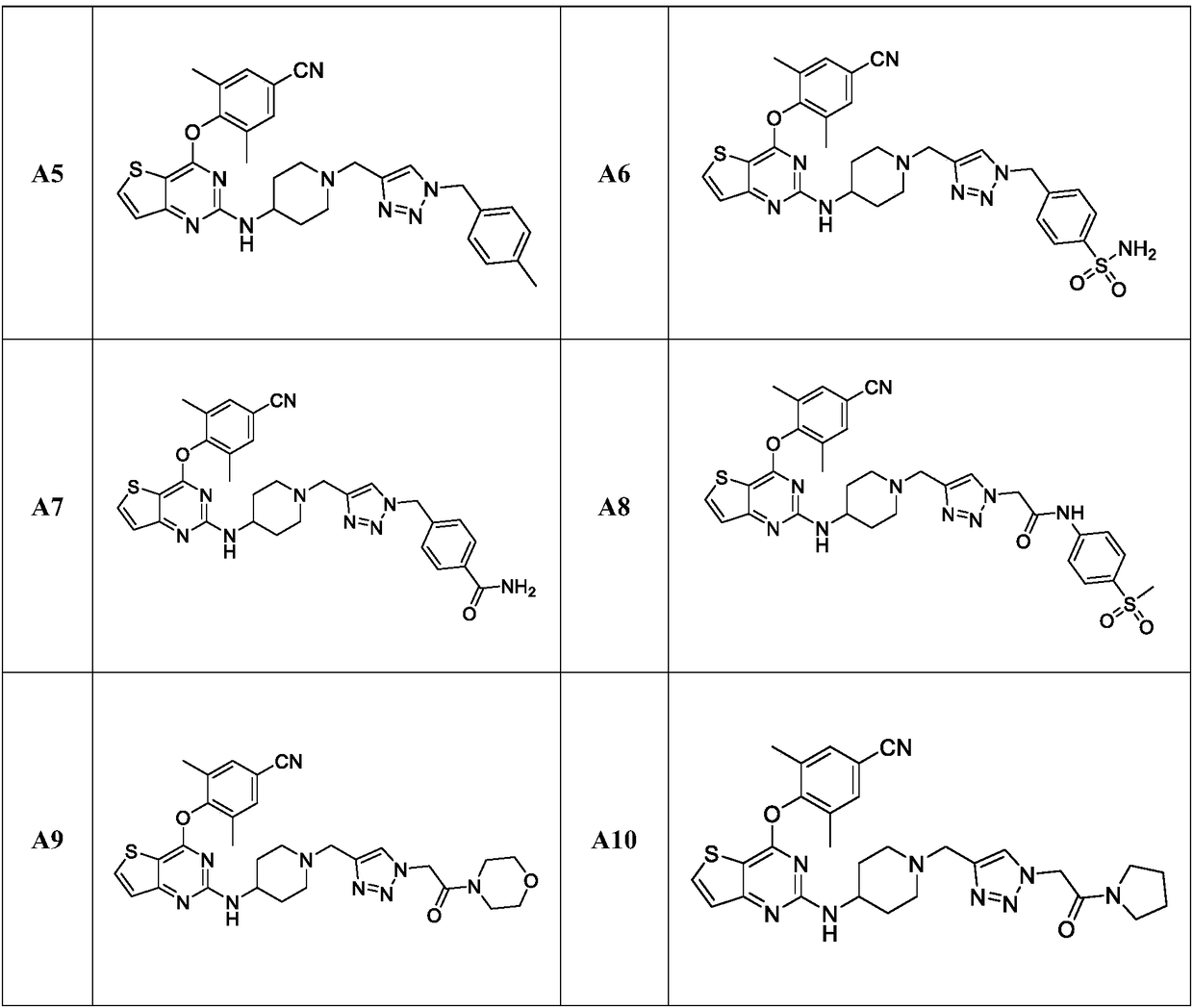
Triazole-ring-containing diaryl pyrimidine HIV-1 inhibitor as well as preparation method and application of triazole-ring-containing diaryl pyrimidine HIV-1 inhibitor
A technology of diarylpyrimidines and HIV-1, which is applied in the field of organic compound synthesis and pharmaceutical application, can solve the problems of drug resistance, toxic and side effects, poor pharmacokinetic properties, etc., and achieve high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of 4-((2-chlorothieno[3,2-d]pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (6)
[0036]
[0037] Weigh 4-hydroxy-3,5-dimethylbenzonitrile (1.5g, 10mmol) and potassium carbonate (1.7g, 12mmol) in 30mL DMF, stir at room temperature for 15 minutes, and then add 2,4-dichlorothiophene And [3,2-d]pyrimidine 1 (1.9g, 10mmol) was stirred at room temperature for 2h (TLC detects the completion of the reaction). When a large amount of white solid is formed, 100 mL of ice water is slowly added to it, filtered, dried in vacuum, and recrystallized from ethanol to obtain Intermediate 6. White solid, yield 93.8%, melting point 258-260°C. ESI-MS: m / z316.3[M+1] + .C 15 H 10 ClN 3 OS(315.02).
Embodiment 2
[0038] Example 2: 3,5-Dimethyl-4-((2-(piperidin-4-ylamino)thieno[3,2-d]pyrimidin-4-yl)oxy)benzonitrile (8) Preparation
[0039]
[0040] 6 (0.95g, 3.17mmol), N-Boc-4-aminopiperidine (0.83g, 3.80mmol) and potassium carbonate (0.87g, 6.33mmol) were added to 20mL of DMF, and then heated to reflux for 10h (TLC Detection). After the reaction was cooled to room temperature, the reaction solution was slowly added dropwise to 50 mL of water, and a large amount of yellow solid was formed. After standing for 30 min, filter and vacuum dry to obtain crude product. Weigh the crude product (1.26g, 2.53mmol) and dissolve it in 4mL of dichloromethane, add 2.22mL of trifluoroacetic acid (30mmol), and stir at room temperature for 3-5h (TLC detection). Then the pH of the reaction solution was adjusted to 9 with saturated sodium bicarbonate solution, extracted with dichloromethane (3×5 mL), washed with saturated sodium chloride solution, and the organic layer was dried with anhydrous sodium sulfa...
Embodiment 3
[0041] Example 3: 3,5-Dimethyl-4-((2-((1-(prop-2-yn-1-yl)piperidin-4-yl)amino)thieno[3,2-d ]Pyrimidine-4-yl)oxy)benzonitrile (9)
[0042]
[0043] Weigh Intermediate 8 (0.5mmol, 0.19g) in 5mL DMF, then add anhydrous potassium carbonate (1.0mmol, 0.14g) and bromopropyne (0.6mmol, 0.07g) in sequence, and react at room temperature (TLC detection The reaction is complete). After the reaction was completed, 20 mL of saturated brine was added to the reaction solution, washed with ethyl acetate (3 x 15 mL), the organic layer was dried with anhydrous sodium sulfate, filtered, and concentrated. Fast column chromatography separation, and then recrystallization from ethyl acetate-petroleum ether to obtain the target compound 9. 1 H NMR(400MHz, DMSO-d 6 )δ8.20(d,J=5.3Hz,1H,C 6 -thienopyrimidine-H),7.72(s,2H, C 3 ,C 5 -Ph-H), 7.27(s, 1H, C 7 -thienopyrimidine-H), 6.88(s,1H,NH), 3.69(s,1H), 3.22(s,2H, N-CH 2 ), 3.13 (s, 1H, CH ≡), 2.74 (s, 2H), 2.12 (s, 6H), 1.90-1.30 (m, 6H). 13 C NMR(100MHz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com