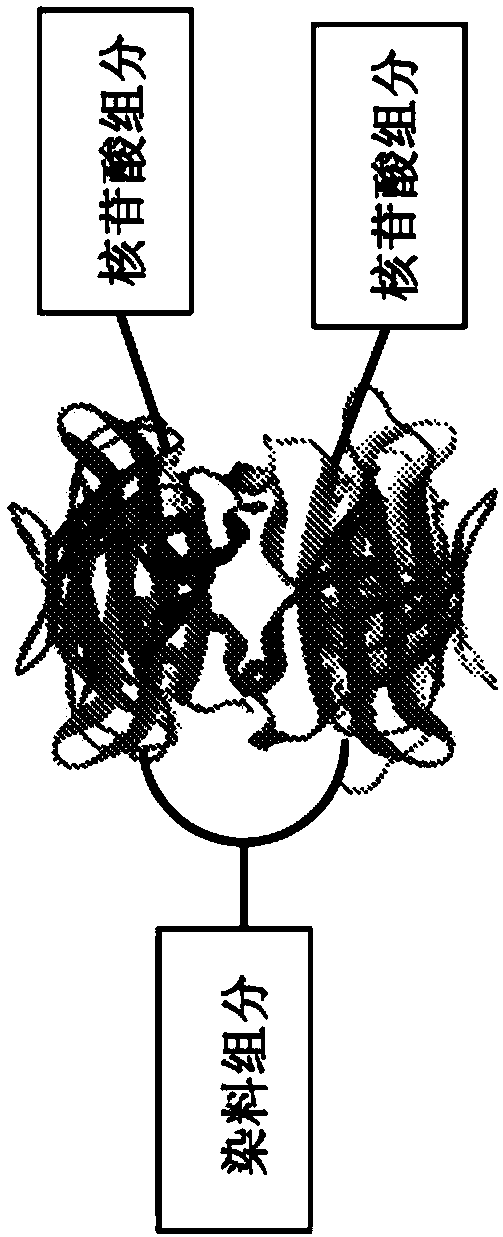Modified nucleotide reagents
A nucleotide and compound technology, used in biological testing, microbial determination/inspection, fluorescence/phosphorescence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0406] Embodiment 1. Synthesis of double biotin nucleotide compounds
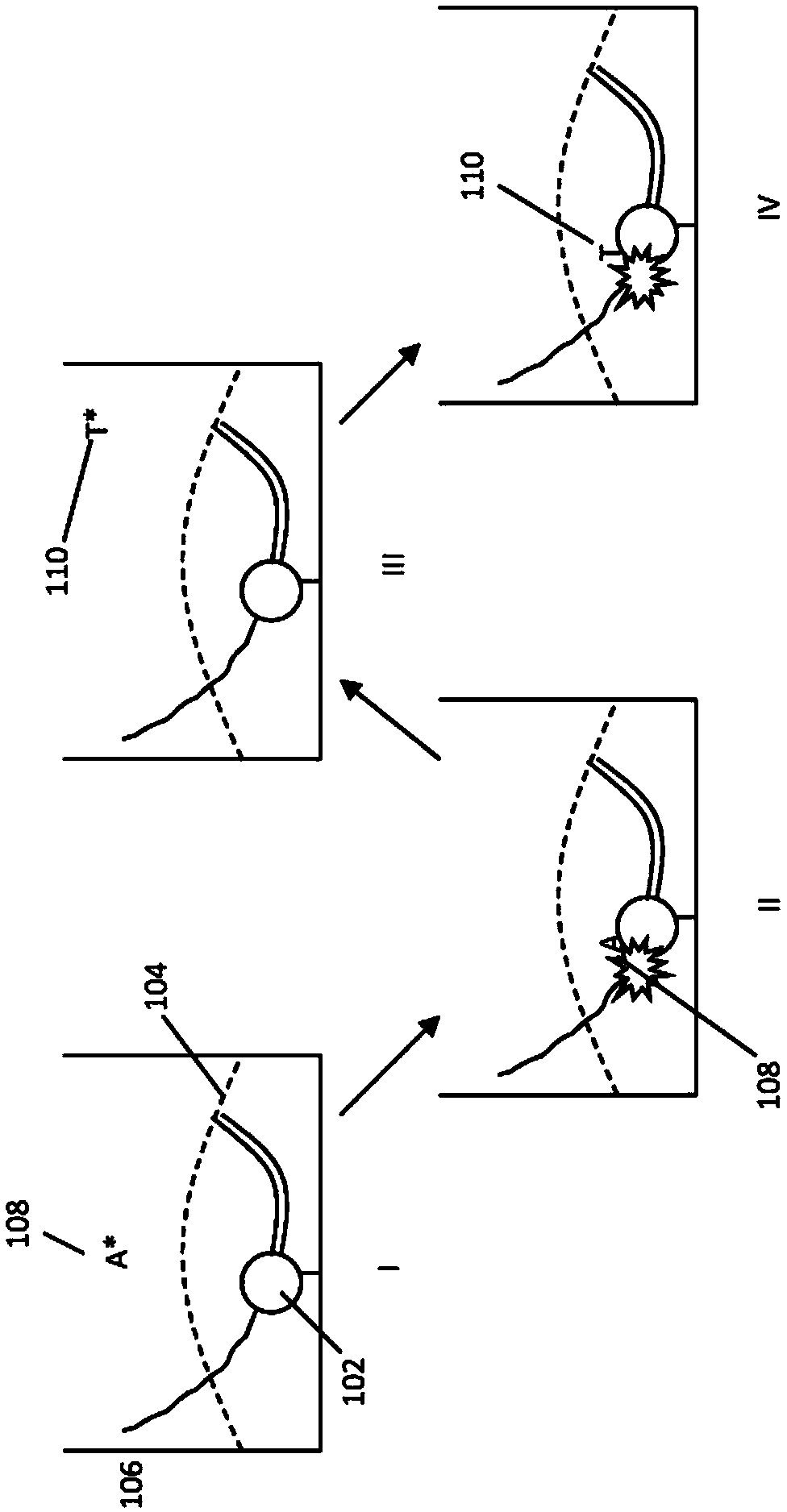
[0407] Various nucleotide compounds containing dual biotin linkers have been synthesized for use in single-molecule real-time sequencing reactions. Avidin proteins have been used to assemble these compounds with dye-labeled compounds, or intermediate forms thereof, also comprising a dual-biotin linker, resulting in dye-labeled nucleotide analog complexes, e.g. as described in Example 2 stated and as Figure 7A to Figure 7D and Figure 7F shown. Additional examples of labeled nucleotide analogs that have been prepared according to these methods are shown schematically in Figure 3E to Figure 3O '. Many analogs exhibit improved photostability, brightness, and reaction kinetics in automated DNA sequencing reactions involving DNA polymerases. See also US Patent Application Publication No. 2013 / 0316912 Al. Example 3 describes the use of assembled fluorescent nucleotide reagent complexes in real-time sequ...
Embodiment 2
[0444] Example 2. Assembly of dye-labeled nucleotide analogs
[0445] The mononucleotide and dinucleotide compounds described above are assembled into dye-labeled Nucleotide analogs. For most of the kinetic experiments described in Example 3, a single avidin protein and a protein such as Figure 4A Simple, unshielded dye-labeled compounds, such as the dye-labeled compounds shown in Figure 1, can be assembled into nucleotide compounds. Such assembly can be performed as described in US Patent Application Publication No. 2013 / 0316912 Al. For example using Figure 7A to Figure 7D and Figure 7F The pathway shown, also assembles more complex analog structures. As described in Example 3, these analogs (e.g. Figure 19A Analogs depicted in ) were evaluated.
Embodiment 3
[0446] Example 3. Use of dye-labeled nucleotide analogs in real-time sequencing reactions
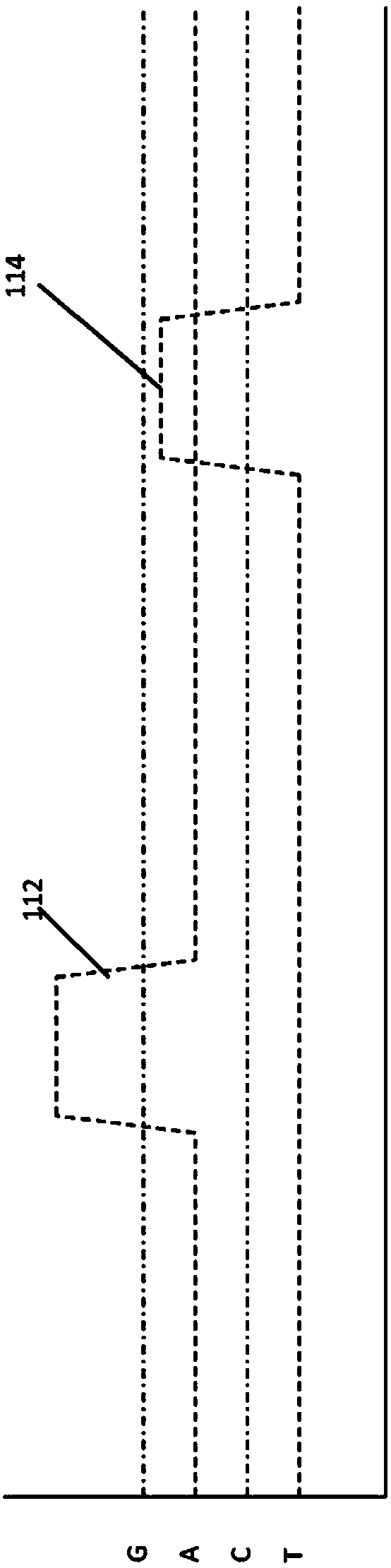
[0447] Single-molecule real-time sequencing reactions using the fluorescent nucleotide analogs described in Example 2 were performed in a zero-mode waveguide ("ZMW") array with 3000 discrete cores. Reactions were visualized using a highly multiplexed confocal fluorescence microscope that provided a directional distribution of illumination, eg, each core as an independent spot. See, eg, US Patent No. 7,714,303, which is hereby incorporated by reference in its entirety for all purposes. Fluorescent signals from various ZMWs were detected using an EMCCD camera, and the signals were subjected to pulse recognition and base calling processes. See, eg, US Patent No. 8,182,993, which is hereby incorporated by reference in its entirety for all purposes. Sequencing was generally performed as described in Eid, J et al. Science 323:133-138 and the corresponding supplementary information include...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com