A kind of goose parvovirus strain and application thereof
A technology for goose parvovirus and goose parvovirus disease, which is applied in the directions of antiviral agents, viruses/phages, viral antigen components, etc., can solve problems such as difficult to protect diseases, and achieve good commercial development prospects, good safety, and effectiveness. The effect of immune protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
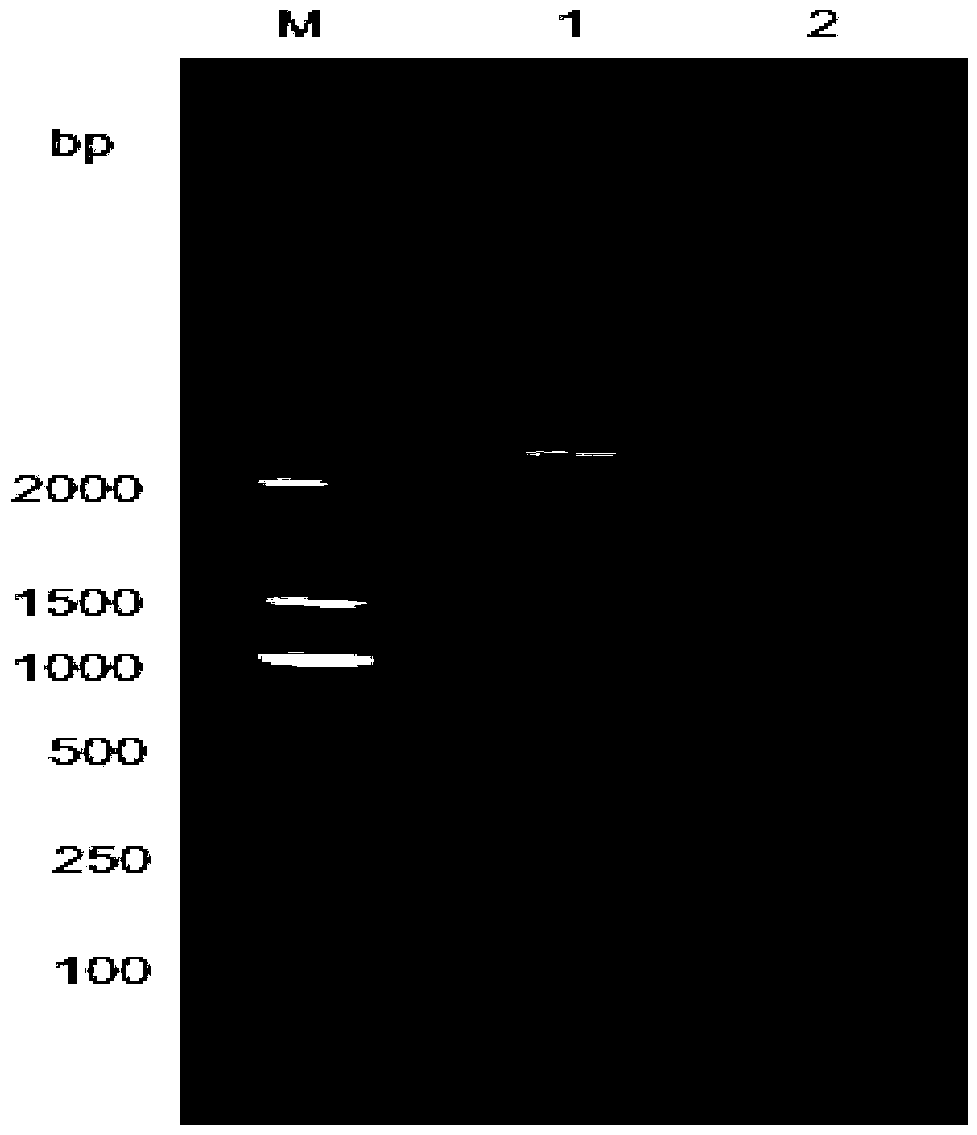
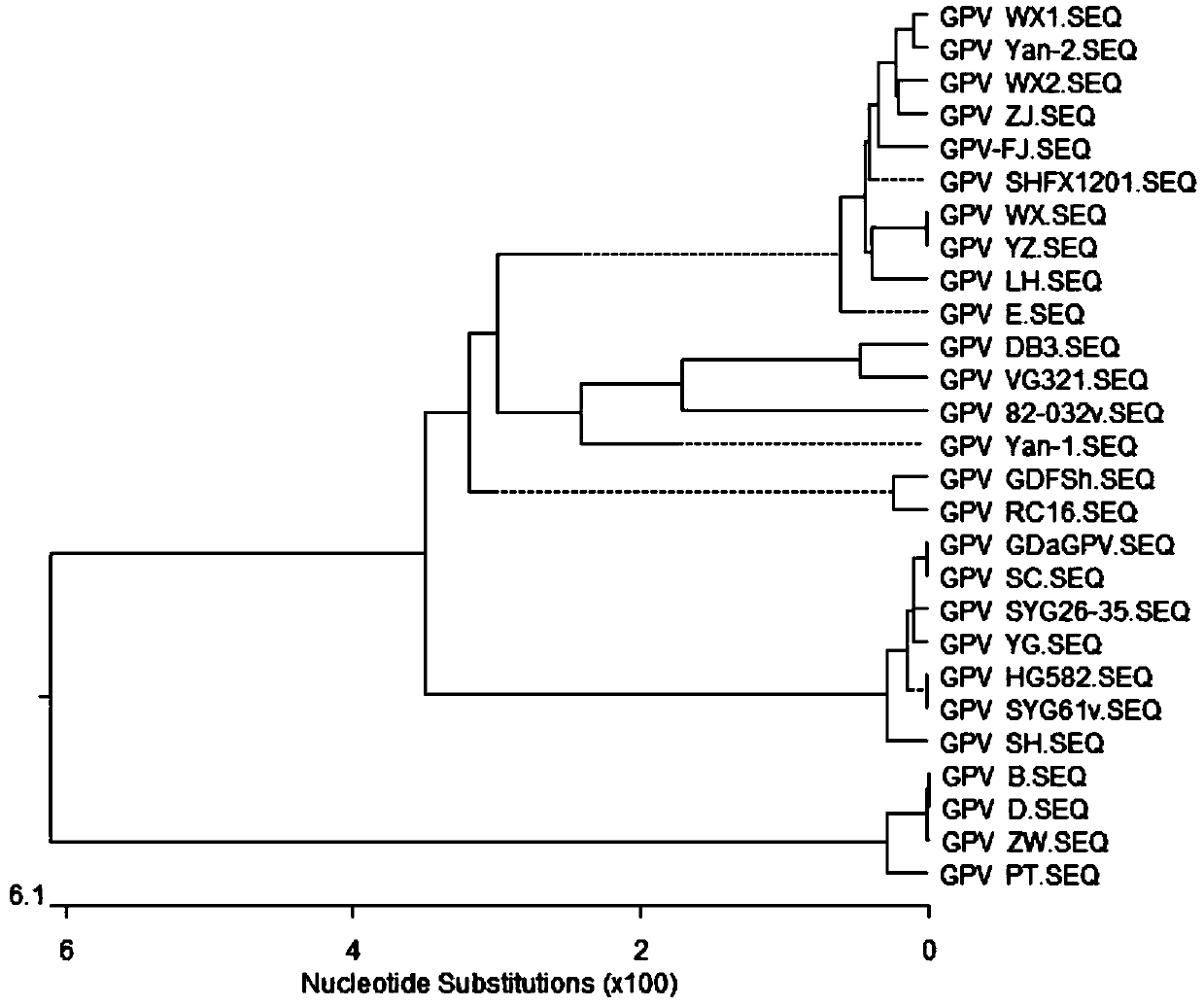
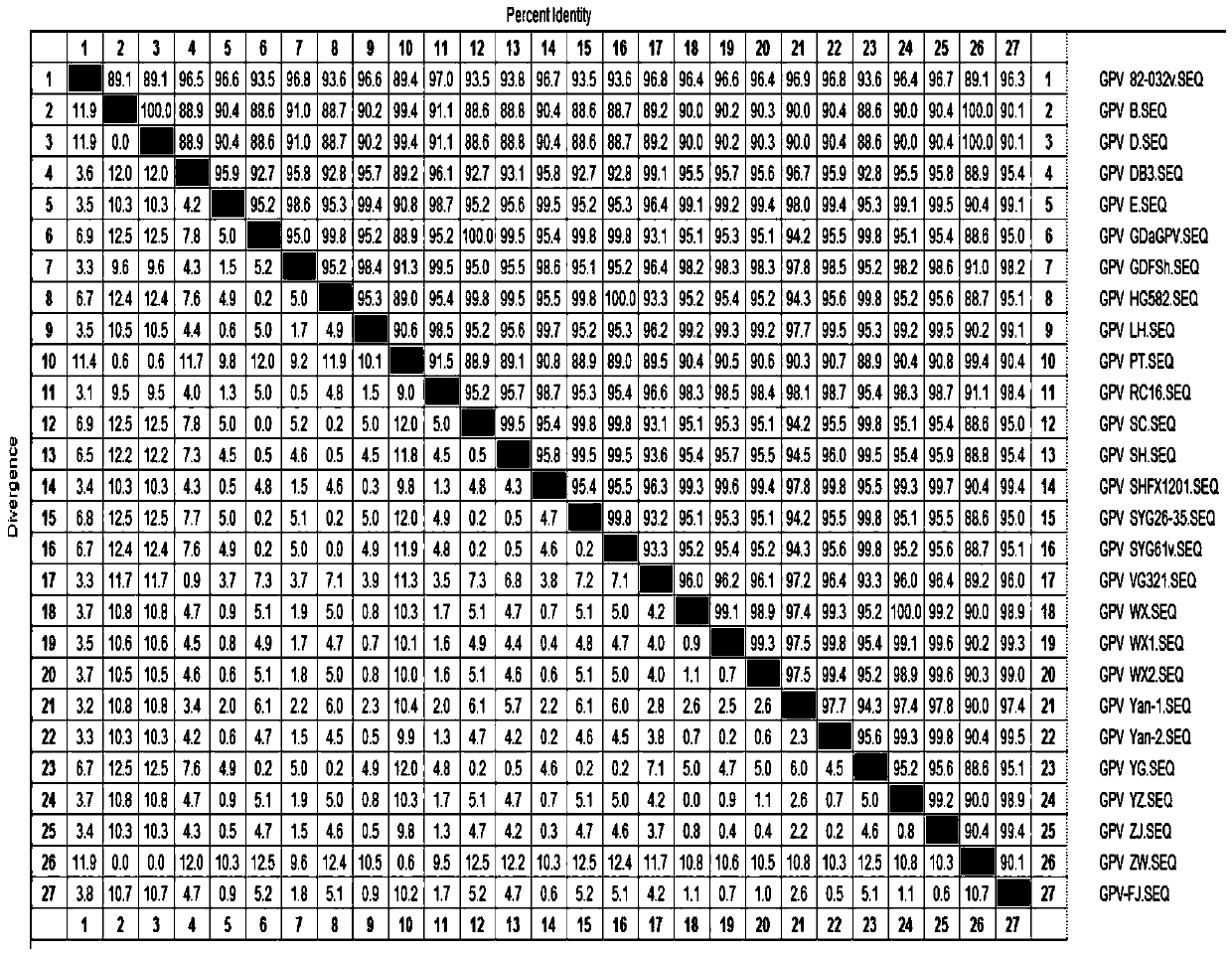
[0016] Isolation and identification of embodiment 1GPV-FJ strain
[0017] 1.1 Epidemiological investigation In May 2017, a group of 8-day-old Muscovy ducks in a Muscovy duck farm in Fujian developed respiratory symptoms, which was the initial stage of the disease. Muscovy duck farms were immunized with commercial duck hepatitis bivalent antibody and goose parvovirus inactivated vaccine at 1 day of age. Feet were soft at 8 days of age, and some ducks died. Antibiotics were used, but the improvement did not improve, and the mortality rate increased day by day. Exudative enteritis and sausage-like emboli formed in the intestinal tract were found to be the main pathological changes in the dead ducks. Systemic dehydration myocardial congestion, liver and spleen enlargement, etc. The laboratory diagnosis was a mutant strain of goose parvovirus.
[0018] 1.2 Virus Isolation Take 50-100 g of diseased tissues such as the brain, viscera, and intestinal tract of the sick white muscovy ...
Embodiment 2
[0039] Embodiment 2 Vaccine manufacture and semi-finished product inspection
[0040] 2.1 Virus seed preparation of GPV-FJ strain The allantoic fluid (the 3rd generation of the original virus seed of GPV-FJ strain) is made 500-fold dilution with sterilized PBS, and inoculates 10 Muscovy duck embryos of 12 days old through the allantoic cavity, each Embryo 0.2ml, place 36~38 ℃ to continue to incubate, select the duck embryo that dies within 48~144 hours after inoculation. The allantoic fluid was collected and centrifuged, and the supernatant was taken for passage to the next generation. According to this method, 10 generations were passed continuously, which were respectively marked as C3-C10 generations. The virus content of each passage was determined. The same generation will be tested for sterility and virus content ≥ 10 5.0 ELD 50 / 0.2ml virus solution, quantitatively aliquoted, freeze-dried and stored.
[0041] 2.2 Preparation of GPV-FJ strain antigen Dilute the viru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com