Alpha-manganese dioxide nanotube, silver nanoparticle loaded alpha-manganese dioxide nanotube, and preparation method and application thereof
A nanoparticle and nanotube technology, applied in the field of air fuel cells, can solve the problem of low catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The present invention provides a kind of α-MnO 2 A method for preparing nanotubes, comprising the steps of:
[0032] (1) Potassium permanganate is mixed with water to obtain potassium permanganate solution;
[0033] (2) carry out reduction reaction after hydrochloric acid is added dropwise in the potassium permanganate solution that described step (1) obtains, obtain α-MnO 2 For nanotubes, the molar ratio of potassium permanganate to hydrochloric acid is 1:1-10.
[0034] The invention mixes potassium permanganate with water to obtain a potassium permanganate solution. The present invention has no special limitation on the concentration of the potassium permanganate solution, and any concentration of potassium permanganate solution can be used. In the present invention, the volume ratio of the mass of potassium permanganate to water is preferably 1.517g:80mL. The present invention has no special limitation on the mixing method, and a mixing method well known to those...
Embodiment 1
[0064] 1.517gKMnO 4 Dissolve in 80mL deionized water, stir evenly, weigh 3.0g 37% HCl and add dropwise to KMnO 4 solution, stirred for 30min.
[0065] Transfer the above solution to a 150mL reactor and carry out the oxidation reaction at 130°C for 10 hours. After the reaction, the reactor was naturally cooled to room temperature, centrifuged, and the precipitate was washed three times with deionized water and ethanol, and the precipitate was vacuum-dried at 100°C. 24h, get α-MnO 2 nanotube.
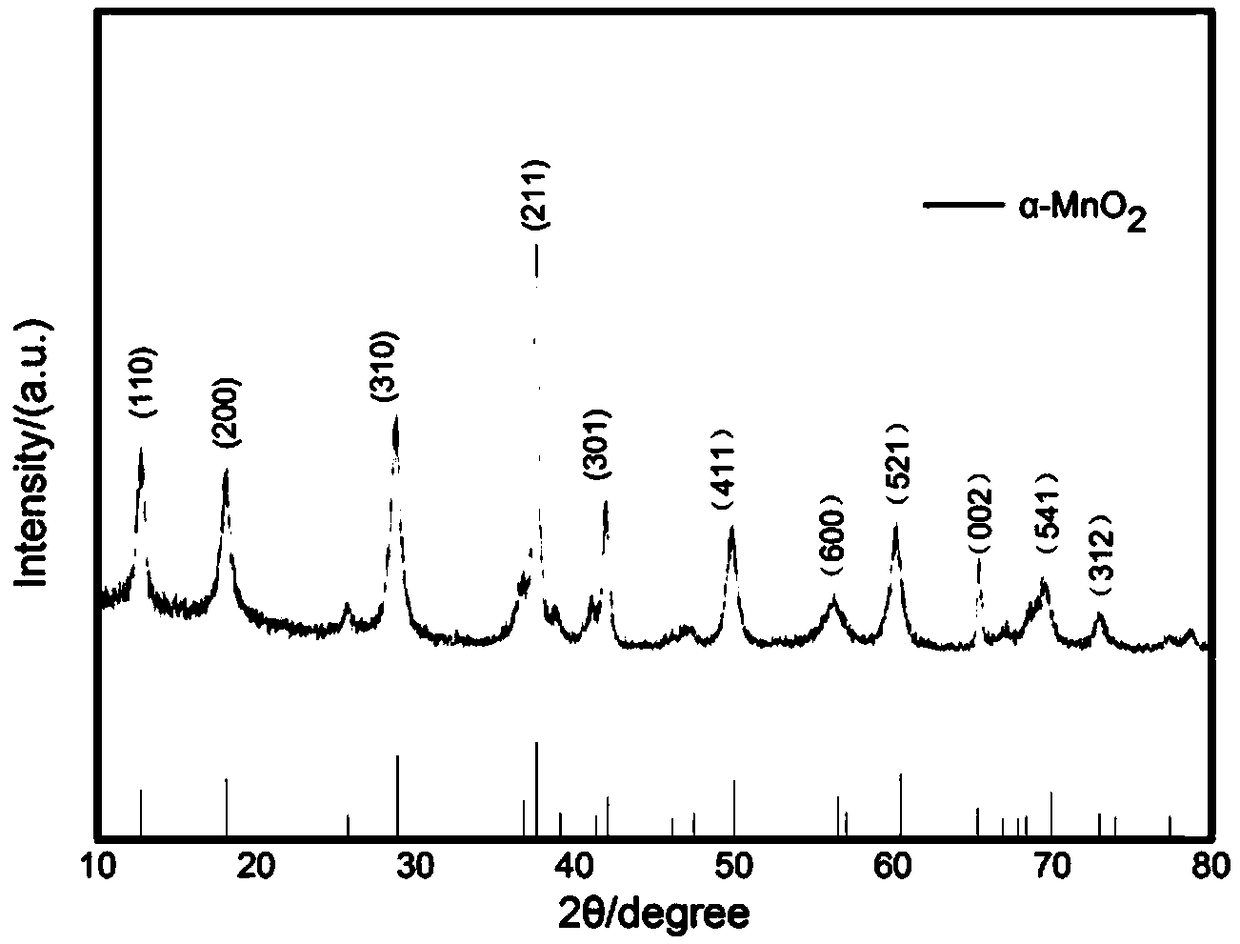
[0066] For the α-MnO prepared in this example 2 The nanotubes were characterized by XRD, and the results were as follows: figure 1 shown by figure 1 It can be seen that the α-MnO prepared in this example 2 Nanotube XRD diffraction patterns and a-MnO 2 (JCPDS No.44-0141, tetragonal, I4 / m, ), it is determined that the synthesized sample is a-MnO 2 square phase.
[0067] For the α-MnO prepared in this example 2 The nanotubes were characterized by SEM and TEM, and the results are ...
Embodiment 2
[0070] The preparation method is the same as that of Example 1, except that the amount of hydrochloric acid added is 2g, 5g and 7g respectively.
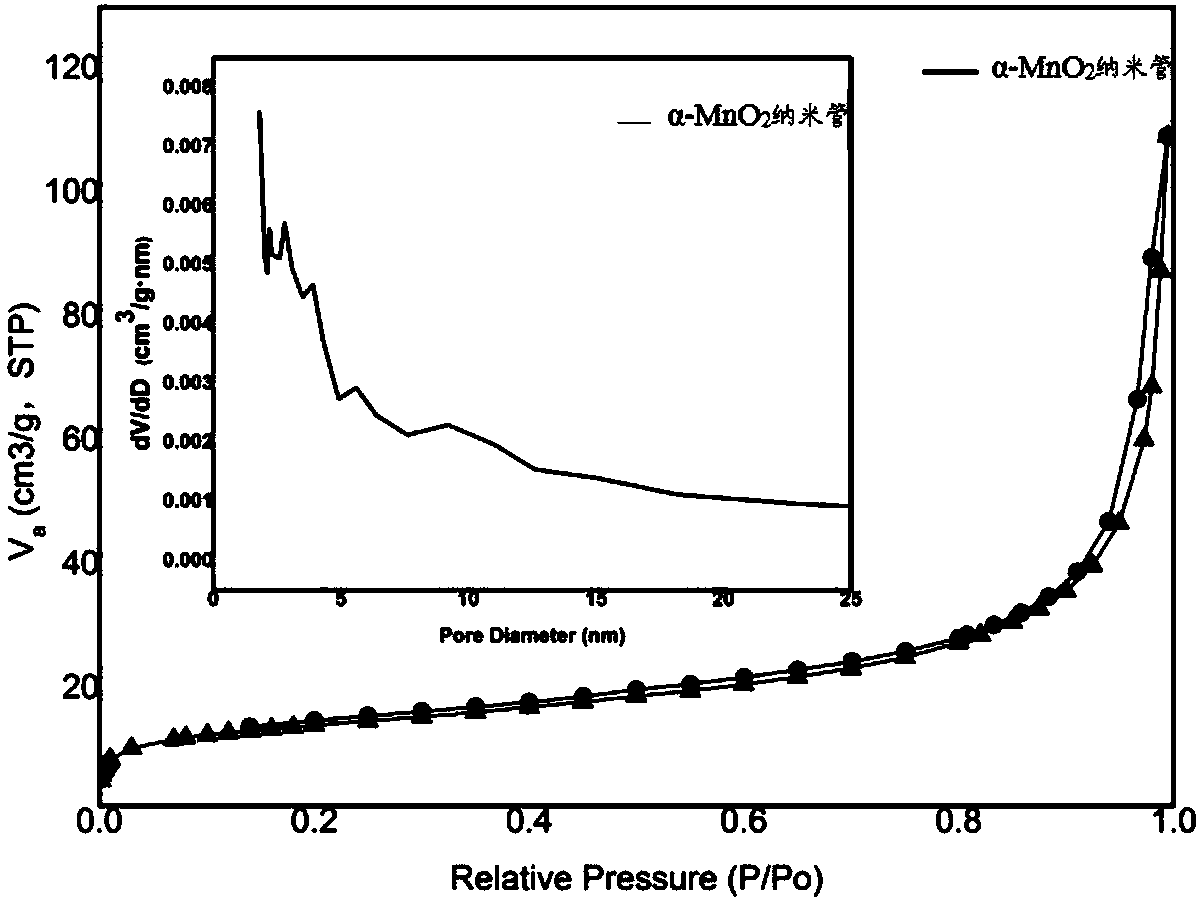
[0071] For the α-MnO that embodiment 1 and 2 make 2 The specific surface area of nanotubes was tested, and the results are shown in Table 1. It can be seen from Table 1 that the addition of hydrochloric acid can adjust the α-MnO 2 The specific surface area of nanotubes, when the added amount is 3g, the BET specific surface area reaches the maximum of 52.9030m 2 / g.
[0072] Table 1 Preparation of α-MnO with different additions of hydrochloric acid 2 BET specific surface area of nanotubes
[0073]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
| Average inner diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com