Method for purifying 1,1,1,2-tetrafluoroethane through purification reaction
A purification method and technology of tetrafluoroethane, applied in chemical instruments and methods, organic chemistry, preparation of halogenated hydrocarbons, etc., can solve uneconomical problems and achieve the effect of solving difficult-to-control and accelerating the transfer of reaction heat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
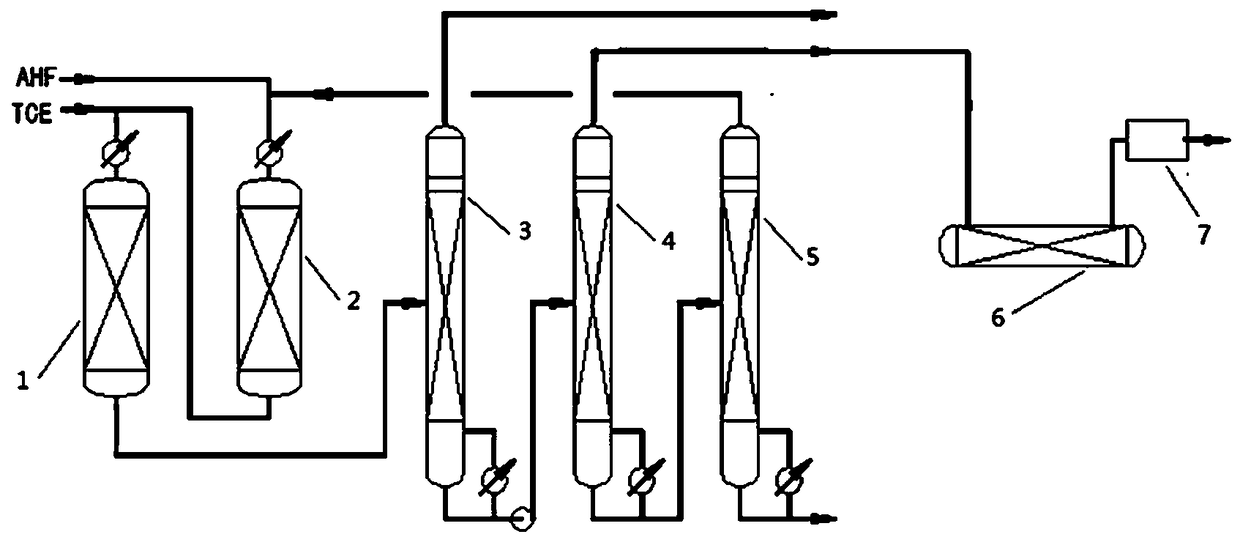
[0025] Trichlorethylene and anhydrous hydrogen fluoride enter the second reactor 2 together, and carry out the second-step reaction at a higher temperature of 350 ° C, and the gas product from the second reactor 2 enters the first reactor together with supplemented trichlorethylene 1. After the first step of the reaction is completed to generate HCFC-133a mixture, the reaction mixture enters the purification reactor 6, and the temperature of the purification reaction is controlled at 180°C.
[0026] The mixed gas entering the purification reactor 6 is: the molar ratio of HF / R134a is 0.25, the R1122 content in the mixed gas is 1500ppm, other olefins are 80ppm, and the hydrogen chloride content is 43% (by mass). The mixed gas is passed into the purification reactor, the reaction pressure is 1.2MPa, and the residence time (t) of the mixed gas is set at 70s.
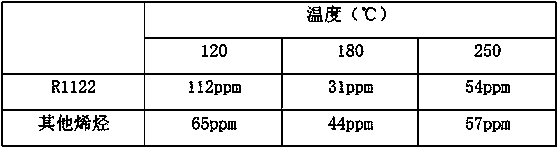
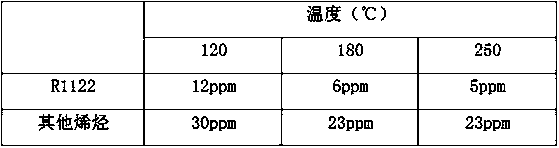
[0027] The material after the purification reaction was tested, and the content of R1122 was 1456ppm, and the total conten...
Embodiment 2
[0029] The material undergoes the second step reaction at a higher temperature of 350°C, and the gas product from the second reactor 2 enters the first reactor 1 together with supplemented trichlorethylene, and the first step reaction is completed to generate HCFC-133a The mixture, the reaction mixture enters the HCl separation tower 3, HCl is removed first, and then the HFC-134a crude product is obtained by separation through the crude product tower 4, and the unreacted HF, HCFC-133a and supplemented HF are sent into the second reactor 2 together, and the remaining gas enters The purification reactor 6 reacts, and the HFC-134a enters the purification device 7 for purification after being catalyzed by the purification reactor 6 to purify and remove olefins by fluorination.
[0030] The mixed gas entering the purification reactor 6 is: the molar ratio of HF / R134a is 0.25, the content of R1122 in the mixed gas is 1500ppm, the content of other olefins is 80ppm, and the content of ...
Embodiment 3
[0035]The material undergoes the second step reaction at a higher temperature of 350°C, and the gas product from the second reactor 2 enters the first reactor 1 together with supplemented trichlorethylene, and the first step reaction is completed to generate HCFC-133a, and the reaction The mixture enters the HCl separation tower 3, HCl is removed first, and then the crude product of HFC-134a is separated by the crude product tower 4, and the unreacted HF, HCFC-133a and supplemented HF are sent to the second reactor 2 together, and the remaining gas enters the purification reactor 6 reaction, HFC-134a enters the purification device 7 for purification after the purification reactor 6 catalyzes the fluorination purification and olefin removal reaction.
[0036] The mixed gas entering the purification reactor 6 is: the molar ratio of HF / R134a is 0.25, the content of R1122 in the mixed gas is 1500ppm, the content of other olefins is 80ppm, and the content of hydrogen chloride is 10p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com