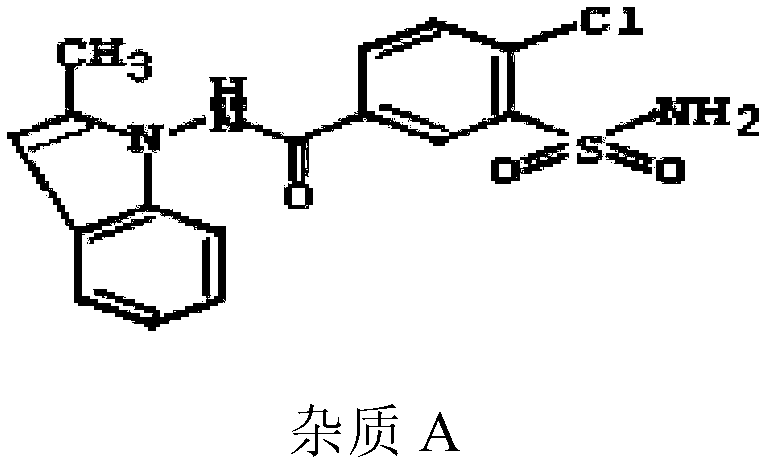
Indapamide tablet and preparation method thereof
A technology of indapamide tablets and indapamide, which is applied in the fields of pharmaceutical formulations, pill delivery, cardiovascular system diseases, etc., can solve the problems of low dissolution rate and high content of genetic impurity A, achieve good dissolution rate and wide application Prospects, the effect of ensuring the quality of preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Present embodiment provides a kind of indapamide tablet, and its prescription (tablet core) is as follows:
[0033] 1 part indapamide,
[0034] 30 parts of cornstarch,
[0035] 5 parts of hydroxypropyl methylcellulose,
[0036] 10 parts of magnesium stearate.
[0037] This embodiment simultaneously provides the preparation method of above-mentioned indapamide tablet, and it comprises the following steps:
[0038] (1) dry-mixing indapamide powder, cornstarch, and hydroxypropyl methylcellulose, then performing wet granulation, and drying to obtain granules;
[0039] (2) adding the granules obtained in (1) to magnesium stearate and blending to obtain the blended granules;
[0040] (3) compressing the blended granules obtained in (2) to obtain plain tablets;
[0041] (4) Prepare a coating solution, and perform a coating operation on the plain tablet obtained in (3) to obtain the indapamide tablet, wherein the temperature during coating is controlled at 40°C.
[0042] ...
Embodiment 2
[0044] Present embodiment provides a kind of indapamide tablet, and its prescription (tablet core) is as follows:
[0045] 1 part indapamide,
[0046] 20 parts of lactose,
[0047] Povidone K30 1 part,
[0048] 5 parts of silicon dioxide.
[0049] This embodiment simultaneously provides the preparation method of above-mentioned indapamide tablet, and it comprises the following steps:
[0050] (1) dry-mixing indapamide powder, lactose, and povidone K30, then performing wet granulation, and drying to obtain granules;
[0051] (2) adding the particles obtained in (1) to silica for total mixing to obtain total mixed particles;
[0052] (3) compressing the blended granules obtained in (2) to obtain plain tablets;
[0053] (4) Prepare a coating solution, and perform a coating operation on the plain tablet obtained in (3) to obtain the indapamide tablet, wherein the temperature during coating is controlled at 38°C.
[0054] After testing, in the indapamide tablets obtained in t...
Embodiment 3
[0056] The present embodiment provides a kind of indapamide tablet, and its prescription (tablet core) is as follows: 1 part of indapamide, 25 parts of microcrystalline cellulose, 5 parts of corn starch, 3 parts of povidone K30, 8 parts of talcum powder share.
[0057] This embodiment simultaneously provides the preparation method of above-mentioned indapamide tablet, and it comprises the following steps:
[0058] (1) dry-mixing indapamide powder, microcrystalline cellulose, and cornstarch, then performing wet granulation, and drying to obtain granules;
[0059] (2) adding the granules obtained in (1) to talcum powder for total mixing to obtain total mixed granules;
[0060] (3) compressing the blended granules obtained in (2) to obtain plain tablets;
[0061] (4) Prepare a coating solution, and perform a coating operation on the plain tablet obtained in (3) to obtain the indapamide tablet, wherein the temperature during coating is controlled at 45°C.
[0062] After testing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com