High-activity coumarin-platinum (II) complex targeting ovarian cancer and synthesis method and application thereof
An ovarian cancer, highly active technology, applied in the direction of organic active ingredients, platinum-group organic compounds, platinum-based organic compounds, etc., to achieve the effect of good aromatic planarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
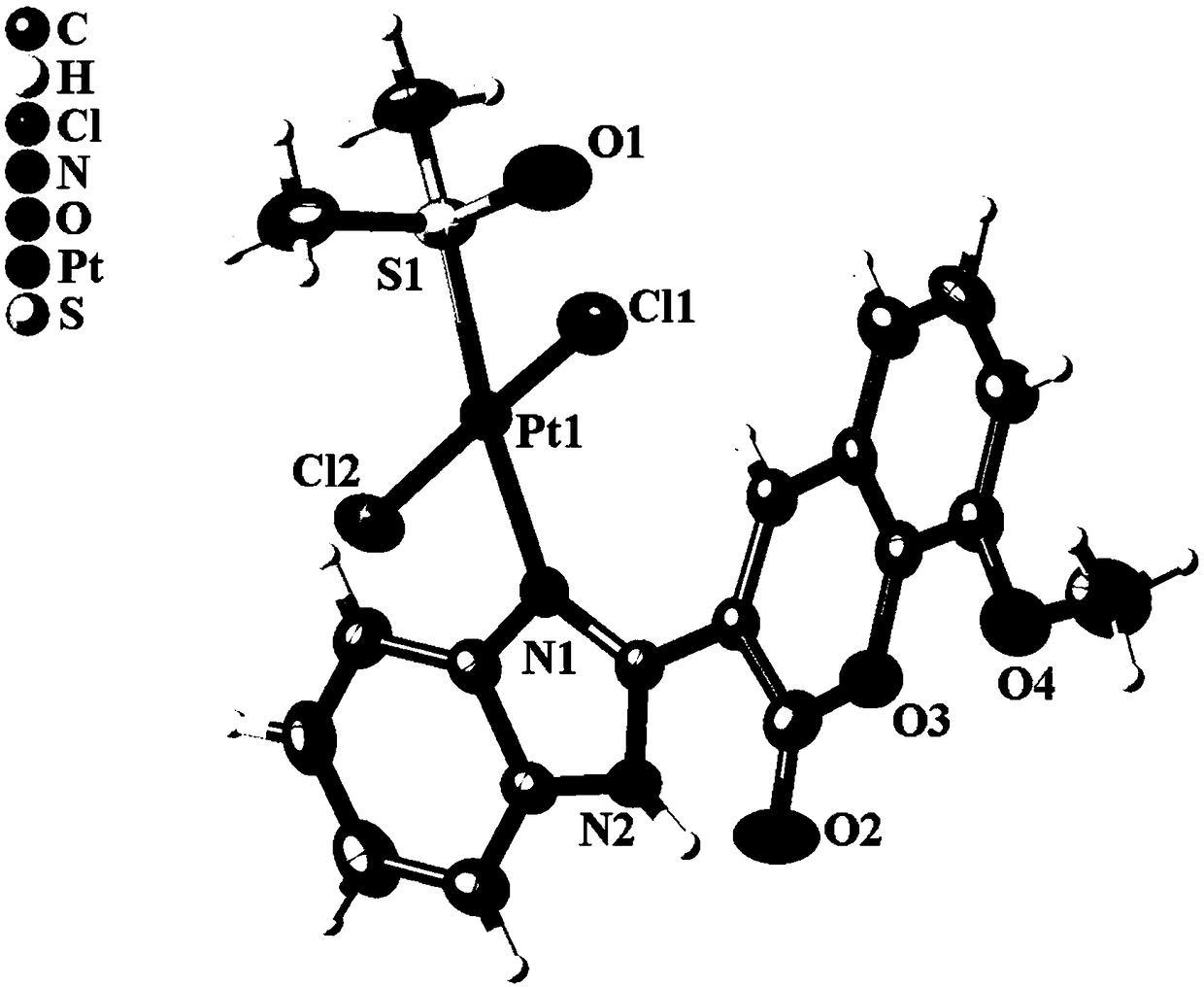
Image
Examples
Embodiment 1
[0045] Accurately weigh the amount of the substance as 1.0mmol dichlorobis(dimethylsulfoxide) platinum (II) and 1.0mmol MeOBC ligand, and dissolve the dichlorobis(dimethylsulfoxide) platinum (II) In 1mL of dimethyl sulfoxide solution, dissolve the MeOBC ligand in 3mL of methanol, then mix the two solutions, react at 90°C for 48 hours, let it stand and cool to room temperature, a yellow blocky solid is precipitated, and the solid is sequentially used After washing with distilled water, methanol and ether, and drying in vacuum, complex 1 was obtained with a yield of 91.3%.
[0046] Identify the resulting yellow blocky crystals:
[0047] (1) Infrared spectrum, its spectrogram is as follows Figure 4 shown.
[0048] IR(KBr):3311,3024,2924,1713,1604,1577,1467,1436,1272,1121,1104,1032,953,778,754,537,438cm -1 .
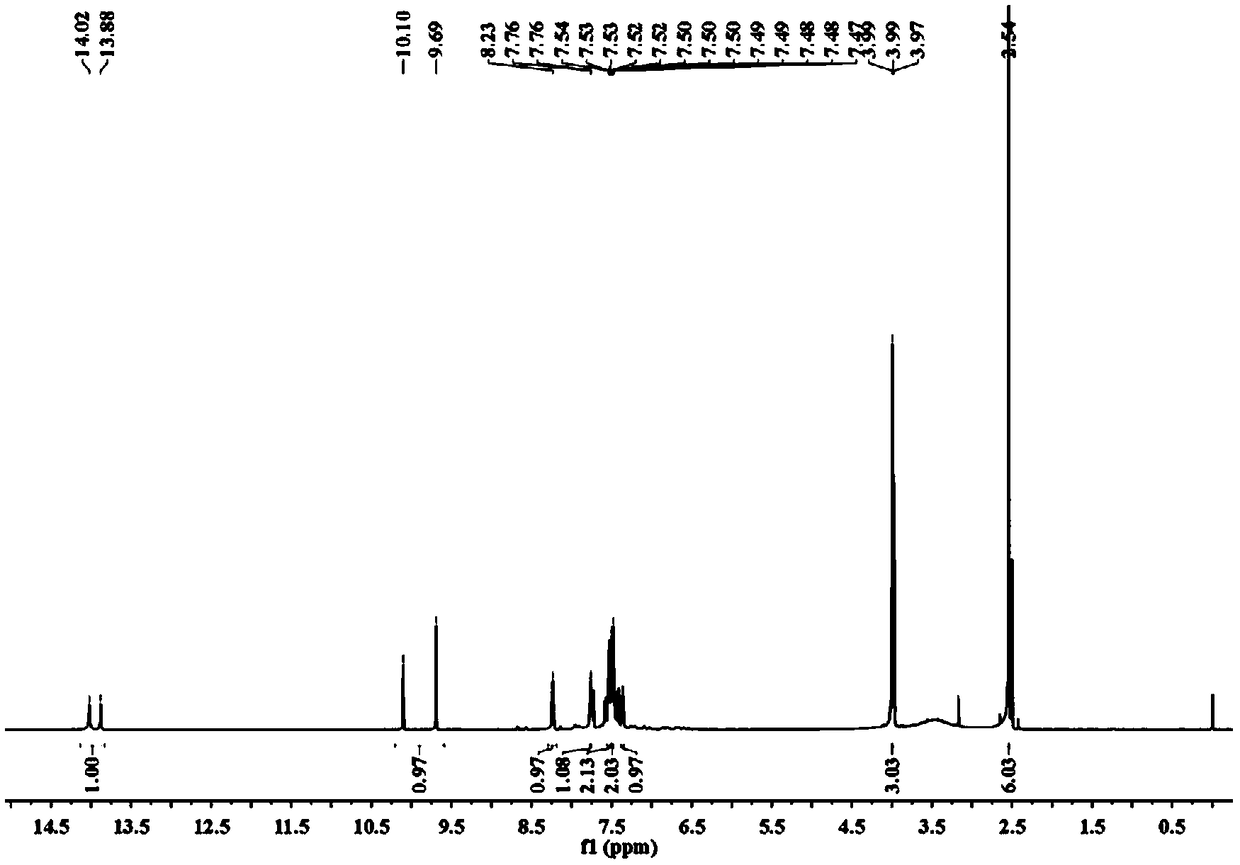
[0049] (2) Proton NMR spectrogram, its spectrogram is as figure 1 shown.
[0050] 1H NMR (600MHz, DMSO-d6) δ13.95 (d, J = 83.9Hz, 1H), 9.90 (d, J = 247.6Hz, 1H), 8.2...
Embodiment 2
[0058] Accurately weigh the amount of the substance as 1.0mmol dichlorobis(dimethylsulfoxide) platinum (II) and 1.0mmol OHBC ligand, dissolve dichlorobis(dimethylsulfoxide) platinum (II) In 1 mL of dimethyl sulfoxide solution, dissolve the OHBC ligand in 4 mL of methanol, then mix the two solutions, react at 90 ° C for 24 hours, let it stand and cool to room temperature, and precipitate a yellow needle-like solid, which is sequentially used After washing with distilled water, methanol and ether, the compound 2 was obtained after vacuum drying with a yield of 85.3%.
[0059] The resulting yellow needle-like solid product is identified:
[0060] (1) Infrared spectrum, its spectrogram is as follows Figure 8 shown.
[0061] IR (KBr): 3325, 3005, 1702, 1606, 1577, 1515, 1435, 1290, 1142, 1092, 1018, 976, 750, 730, 543, 510, 438cm-1.
[0062] (2) Proton NMR spectrogram, its spectrogram is as Figure 5 shown.
[0063] 1H NMR (600MHz, DMSO-d6) δ13.92(d, J=84.1Hz, 1H), 10.62(s, 1...
Embodiment 3
[0071] Accurately weigh 1.0 mmol of dichloro-bis(dimethylsulfoxide) platinum (II) and 1.0 mmol of BC ligand, dissolve dichloro-bis(dimethyl sulfoxide) platinum (II) Dissolve the BC ligand in 3 mL of methanol in 0.5 mL of dimethyl sulfoxide solution, then mix the two solutions, react at 90 ° C for 48 hours, cool to room temperature, and precipitate a yellow granular solid. After washing and vacuum drying, complex 3 was obtained with a yield of 76.22%.
[0072] The obtained yellow granular solid is identified:
[0073] (1) Infrared spectrum, its spectrogram is as follows Figure 12 shown.
[0074] IR (KBr): 3328, 3302, 3005, 1712, 1605, 1572, 1434, 1319, 1140, 1106, 1024, 741, 440cm-1.
[0075] (2) Proton NMR spectrogram, its spectrogram is as Figure 9 shown.
[0076] 1H NMR (600MHz, DMSO-d6) δ13.95(d, J=88.0Hz, 1H), 9.89(d, J=251.9Hz, 1H), 8.23(t, J=7.9Hz, 1H), 7.99(ddd ,J=7.4,5.6,1.3Hz,1H),7.87-7.83(m,1H),7.55(ddd,J=8.2,4.7,0.7Hz,1H),7.49(ddd,J=9.0,5.4,2.8Hz ,2H), 7.42...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com