A broad-spectrum multiple subunit vaccine against Streptococcus suis infection
A streptococcus and vaccine technology, applied in the biological field, can solve the problems of inability to protect multiple serotypes of Streptococcus suis, poor effect, limited antigen expression and exposure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
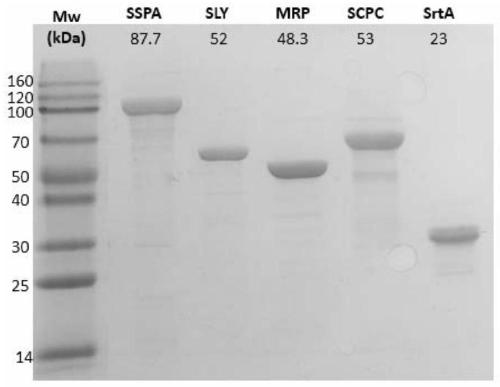
[0103] Embodiment 1, preparation of multiple recombinant protein vaccine V5
[0104] (1) Preparation of recombinant protein
[0105] 1. Preparation of sortase A (SrtA)
[0106] 1. Using the genomic DNA of Streptococcus suis type 2 (A7) as a template, perform PCR amplification with a primer pair composed of F1 and R1 to obtain a PCR amplification product.
[0107] F1: 5'-CATGCCATGGCCAGCAATGTTACGACAGA-3';
[0108] R1: 5'-CCGCTCGAGTTGTCCATAATCATACTGAT-3'.
[0109] 2. Use restriction endonucleases NcoI and XhoI to double digest the PCR amplification product in step 1, and recover the digested product.
[0110] 3. Digest the vector pET28a(+) with restriction endonucleases NcoI and XhoI, and recover the vector backbone of about 5400bp.
[0111] 4. Ligate the digested product of step 2 with the vector backbone of step 3 to obtain the recombinant plasmid pET28a-SrtA. According to the sequencing results, the results of the recombinant plasmid pET28a-SrtA are described as follows: ...
Embodiment 2
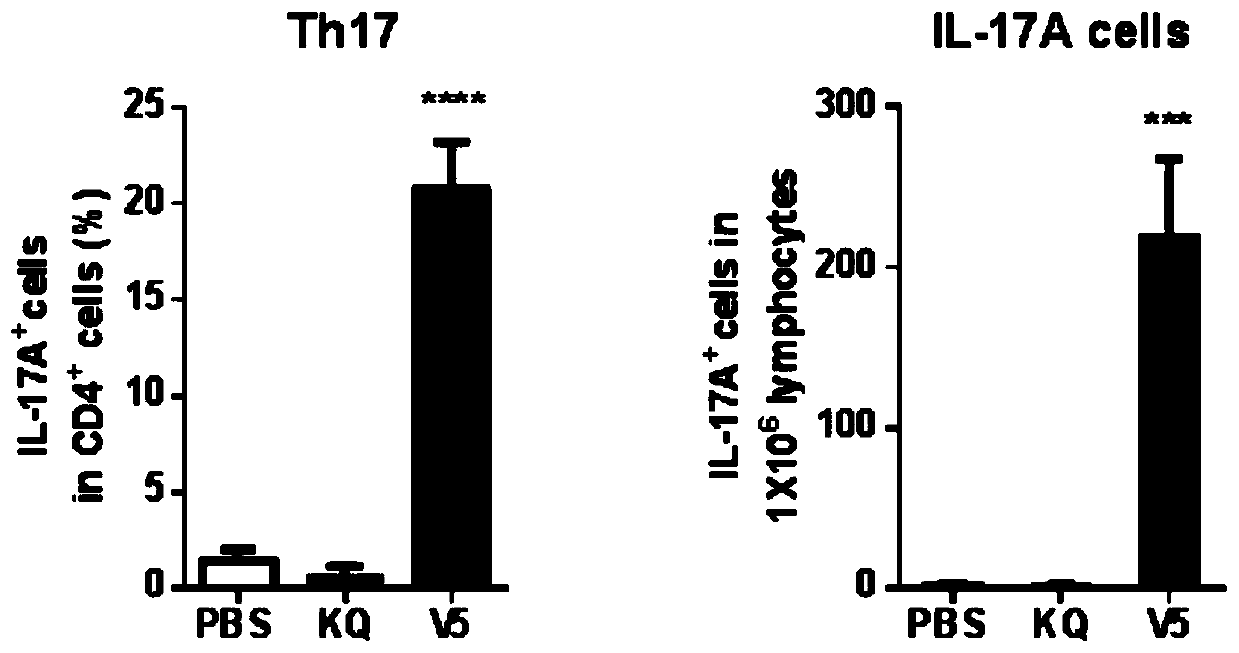
[0173] Example 2, Nasal Immunization V5 Induces Th17 Cell Response
[0174] Four-week-old female C57BL / 6J mice were randomly divided into three groups, and the groups were treated as follows:
[0175] PBS group: On the 0th day, the 7th day and the 14th day of the experiment, 10 μl of PBS buffer was instilled through the nasal cavity, and each mouse was given 10 μl each time.
[0176]V5 group: on the 0th day, the 7th day and the 14th day of the experiment, the V5 vaccine solution was instilled through the nasal cavity respectively, and each mouse was given the V5 vaccine solution each time (the V5 vaccine solution was composed of 10 μg, 10 μg CpG and 10 μg of the five recombinant proteins respectively). 10 μl of PBS buffer obtained by mixing).
[0177] KQ group: on the 0th day, the 7th day and the 14th day of the experiment, the commercially available pre-inactivated vaccine (KQ for short) was injected intramuscularly, and the concentration of the bacterial solution was 1.5×10...
Embodiment 3
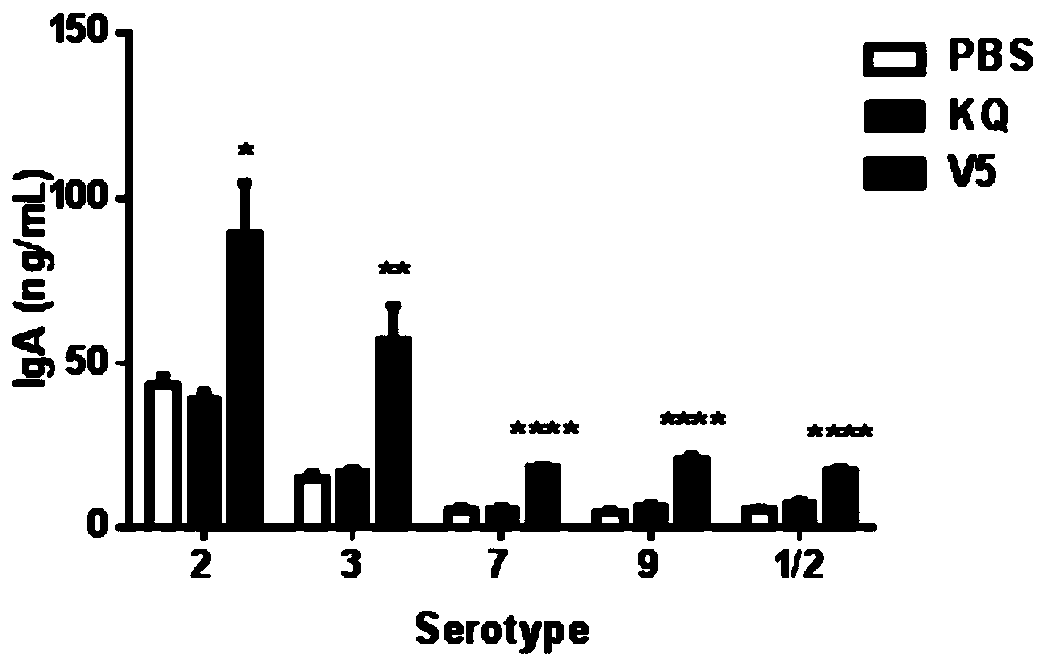
[0180] Example 3, V5 immunization induced antibody response in mice
[0181] Four-week-old female C57BL / 6 mice were randomly divided into three groups, and the groups were treated as follows:
[0182] PBS nasal drop group: on the 0th day, 7th day and 14th day of the experiment, PBS buffer was instilled through the nasal cavity respectively, and each mouse was given 10 μl each time.
[0183] V5 group: on the 0th day, the 7th day and the 14th day of the experiment, the V5 vaccine solution was instilled through the nasal cavity respectively, and each mouse was given the V5 vaccine solution each time (the V5 vaccine solution was composed of 10 μg, 10 μg CpG and 10 μg of the five recombinant proteins respectively). 10 μl of PBS buffer obtained by mixing).
[0184] KQ group: on the 0th day, the 7th day and the 14th day of the experiment, the commercially available pre-inactivated vaccine (KQ for short) was injected intramuscularly, and the concentration of the bacterial solution wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com