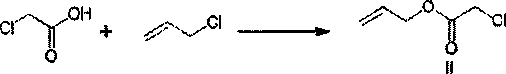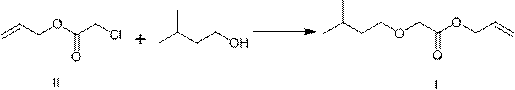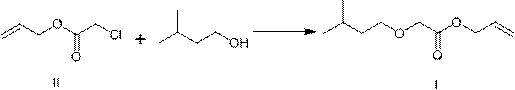Allyl amyl glycolate preparation method
A compound, the technology of chloroacetic acid, applied in the field of preparation of glycopyrrolate, can solve the problems of high hazard, high production cost, and difficult quality control, etc., and achieve the effect of reducing hazard, production cost and solid waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 compound II
[0032]
[0033] In a three-necked flask equipped with a stirring bar, add 120ml of N,N-dimethylformamide, add 8.6g of sodium hydroxide, then add 16.4g of chloroacetic acid and 18.6g of allyl chloride, and react at room temperature for 3~4h, After the reaction was completed, part of the DMF was removed by rotary evaporation, then diluted with water, extracted with dichloromethane, the organic layers were combined and concentrated to obtain 20.8 g of compound II, with a yield of 89%.
Embodiment 2
[0034] Preparation of Example 2 Compound I (Gaponate)
[0035]
[0036] Add 185g of isoamyl alcohol and 12.6g of sodium hydroxide into a three-necked flask equipped with a stirring bar and a thermometer, heat to 130~140°C for reflux dehydration for 4 hours, then cool to room temperature, slowly add 18.2g of compound II, and distill and recover isoamyl alcohol after the reaction is complete. Amyl alcohol, then acidified with dilute hydrochloric acid to pH = 6~7, separated the organic layer, and distilled to obtain 22.9 g of colorless and transparent liquid Gelponate, with a yield of 91%. HPLC: 99.5%.
Embodiment 3
[0037] Example 3 Preparation of Compound II
[0038]
[0039] In a three-neck flask equipped with a stirring bar, add 110ml of N,N-dimethylformamide, add 6.0g of sodium hydroxide, then add 14.2g of chloroacetic acid and 11.5g of allyl chloride, and react at room temperature for 3~4h, After the reaction was completed, part of the DMF was removed by rotary evaporation, then diluted with water, extracted with dichloromethane, the organic layers were combined and concentrated to obtain 16.2 g of Compound II, with a yield of 80.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com