Combination therapy
A phenyl and benzyloxy technology, applied in the field of combination therapy, can solve problems such as poor prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
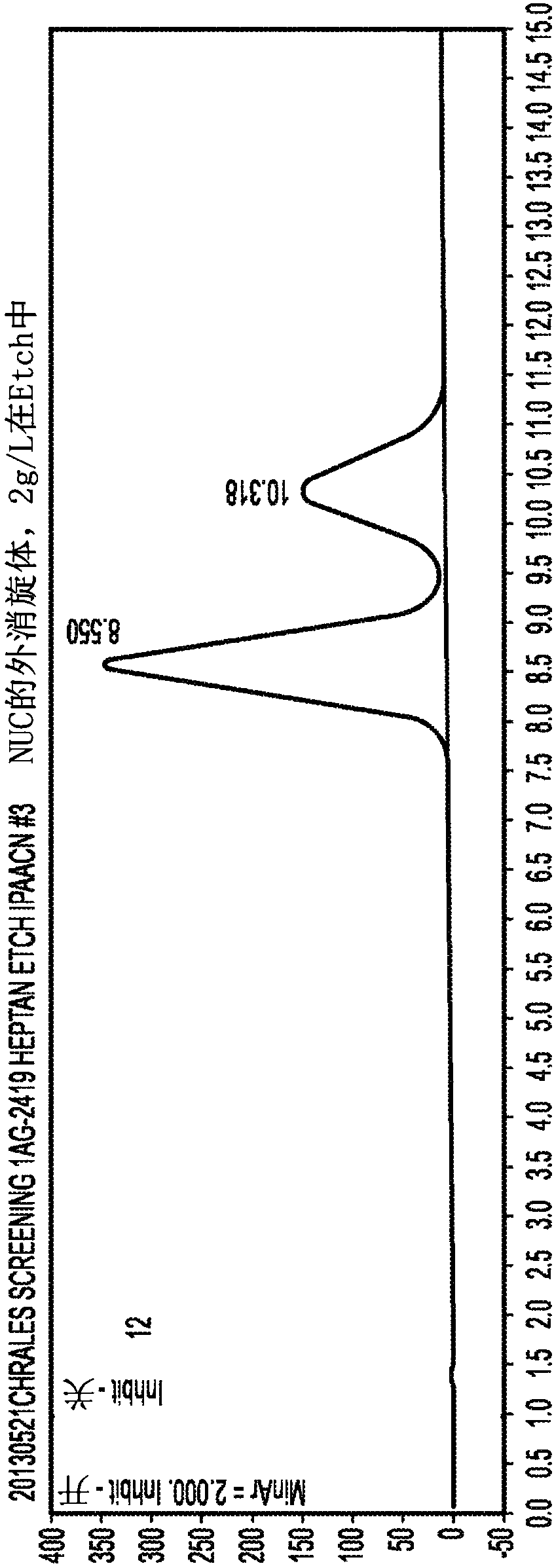
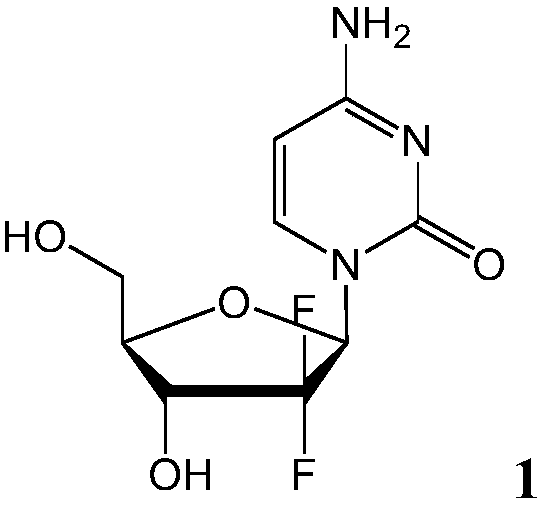
[0192] Example 1 - Single Diastereomer of NUC-1031
[0193] The (R) and (S) isomers can be separated by HPLC under the following conditions:
[0194] Equipment: Agilent 1200 with DAD detector TM series
[0195] Flow rate: 1.0mL / min
[0196] Column: Chiralpak AD TM ;250×4.6mm ID (positive phase)
[0197] Temperature: Ambient
[0198] Particle size: 20μm
[0199] Feed: soluble in methanol; 10g / L
[0200] Solvent: n-heptane / IPA10->50% isopropanol
[0201] Chromatograms such as figure 1 shown. The (S)-epimer eluted at 8.6 minutes and the (R)-epimer at 10.3 minutes.
[0202] Characterization methods and materials: Protons were recorded at 25°C on a Bruker Avance500 spectrometer ( 1 H), carbon ( 13 C), phosphorus ( 31 P) and fluorine ( 19 F) NMR spectrum. Spectra are automatically calibrated to deuterated solvent peaks, all 13 C NMR and 31 P NMR homogeneous proton decoupling. Using Varian Polaris C18-A (10 μM) as the analytical column, using H from 100 / 0 to 0 / 100 w...
Embodiment 2
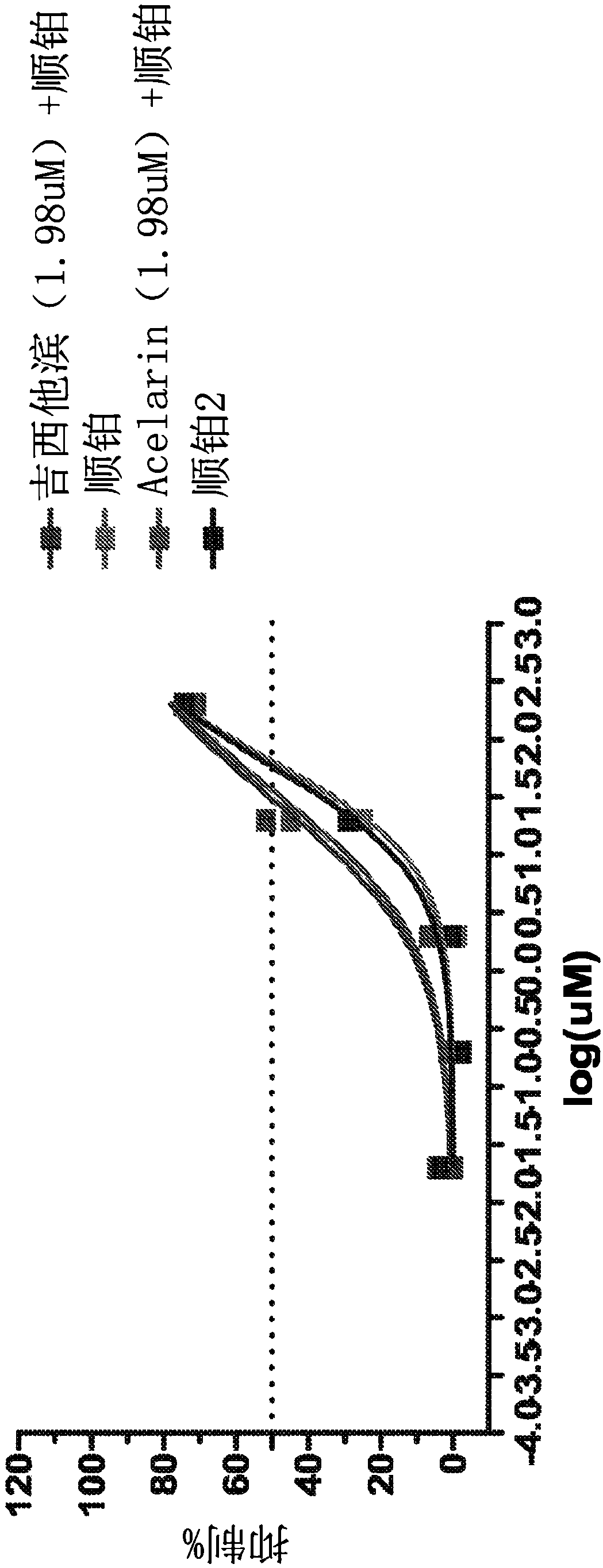
[0217] Example 2 - In vitro study of NUC-1031 and cisplatin combination.
[0218] 2.1 Materials and methods
[0219] Cell Culture and Reagents
[0220] A2780, SK-OV-3, OVCAR-3, NCI-H460, NCI-H1975, NCI-H2122, 5637 and HT1376 in RPMI medium 1640 (Invitrogen-10099141) supplemented with 10% fetal bovine serum (FBS; Invitrogen-10099141) 22400105) in culture. Keep all cell lines at 37°C, 5% CO 2 in a humid incubator. Cell culture media and supplements were purchased from Invitrogen, tissue culture flasks were purchased from Corning, and 96-well and 384-well plates were purchased from Greiner. The CellTiter-Glo Luminescent Cell Viability Assay Kit was purchased from Promega (Promega-G7573), the cell counter Vi-Cell was purchased from Beckman, and the detection instrument Envision was purchased from PerkinElmer. Paclitaxel (used as reference) and cisplatin were purchased from SELLECK with the highest purity. All compounds were soluble in DMSO and when diluted into media. DMS...
Embodiment 3
[0270] Example 3 - Further in vitro studies of the combination of NUC-1031 and cisplatin
[0271] Cell Culture and Reagents
[0272] SKOV3 cells were purchased from the American Type Culture Collection (ATCC). Place them in a 10% Gibco TM FBS (Life Technologies; 10270-106) and 1% Gibco TM Penicillin-Streptomycin (Life Technologies; 15140122) Gibco TM RMPI Medium 1640+GlutaMAX TM -I (Life Technologies; 61870-010) (called complete medium), at 37°C, 5% CO 2 Cultured in cell culture flasks. Cisplatin was purchased from TEVA UK Limited (PL 00289 / 1146). Fluvastatin and Sulforaphane were both purchased from Merck Millipore Corporation (catalog numbers 344096 and 574215, respectively). (S)-NUC-1031 by Ltd provided.
[0273] Cell culture for drug application
[0274] SKOV3 cells were plated at 250 cells in 200 μl per well on In the middle 60 wells of the 3596 96-well plate, each well of the outer 36 wells was filled with 200 μl of PBS. Cells were grown in complete med...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com