Method for increasing dispersion stability of nanoparticles as T1 MRI contrast agent and T1 MRI contrast agent nanoparticles
A technology of dispersion stability and nano-particles, which is applied in the direction of nuclear magnetic resonance/magnetic resonance imaging contrast agents, pharmaceutical formulations, preparations for in vivo tests, etc. spin, low surface area-to-volume ratio effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Example 1: Synthesis of dextran nanocarriers and partial coating of T1-type contrast materials
[0103] Dextran nanocarriers were synthesized by crosslinking of dextran (molecular weight of 10 kDa, Pharmacosmos, Denmark). Specifically, after dissolving 1.8 g of dextran in an alkaline aqueous solution, epichlorohydrin (6 mL of epichlorohydrin, Sigma, USA) and ethylenediamine (26 mL of ethylenediamine, Sigma, USA) were added as a crosslinking agent. ), at room temperature, reacted 24 hours in a constant temperature tank. Reactants were purified using hollow fiber membrane filters (MWCO 10000, GE Healthcare, Netherlands). The synthesized dextran nanocarriers exhibited a hydrodynamic diameter of about 4.1 nm. FeCl2, FeCl3 and NaOH were added to the synthesized dextran nanocarrier at a ratio of 1:2:8 mol, and stirred under strong magnetic force at room temperature for 30 minutes, and Fe3O4 was introduced into the T1 contrast material. Synthesized nanoparticles were purifi...
Embodiment 2
[0104] Embodiment 2: the ratio adjustment of the T1 type contrast material attached to the dextran nanocarrier
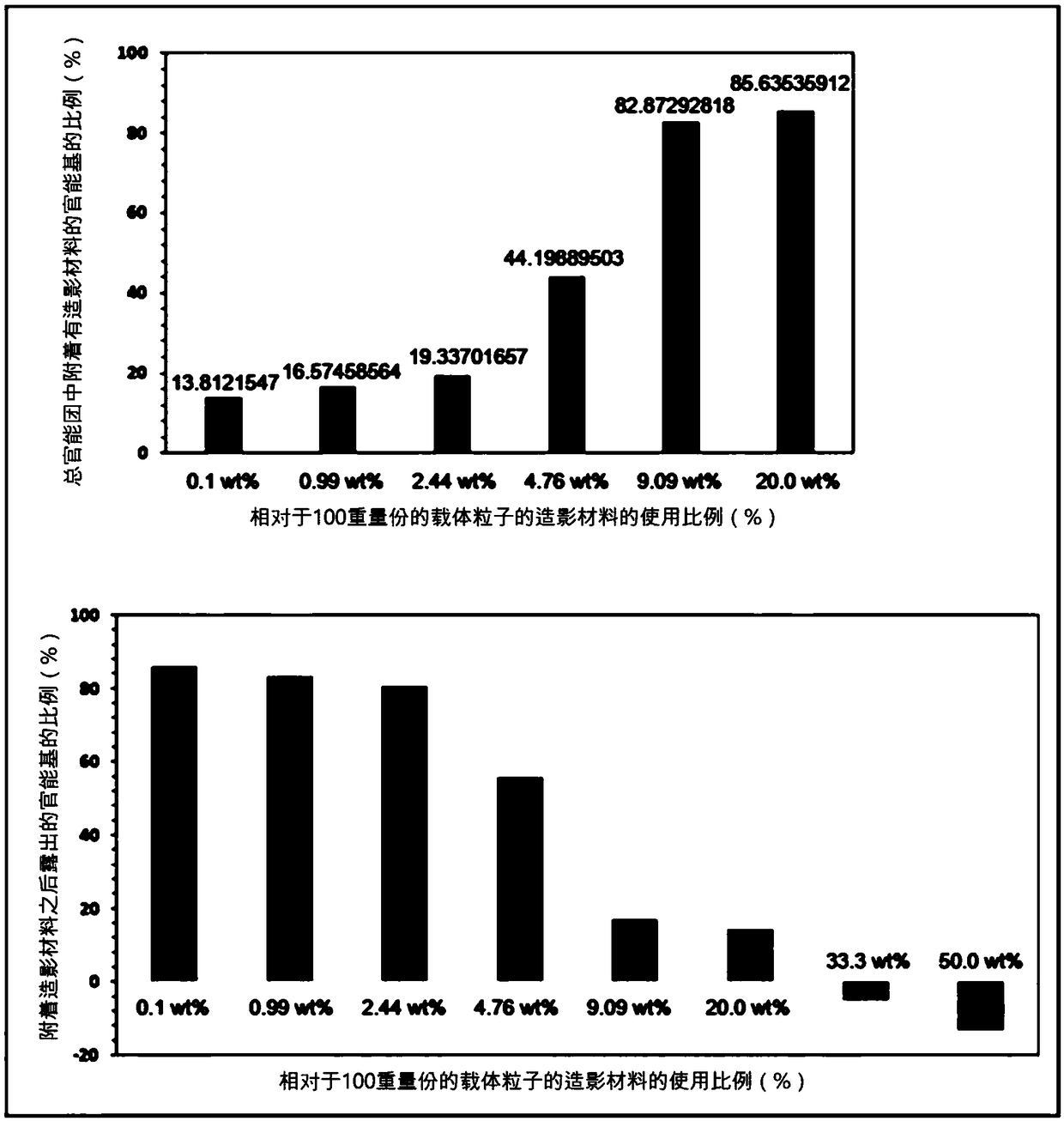
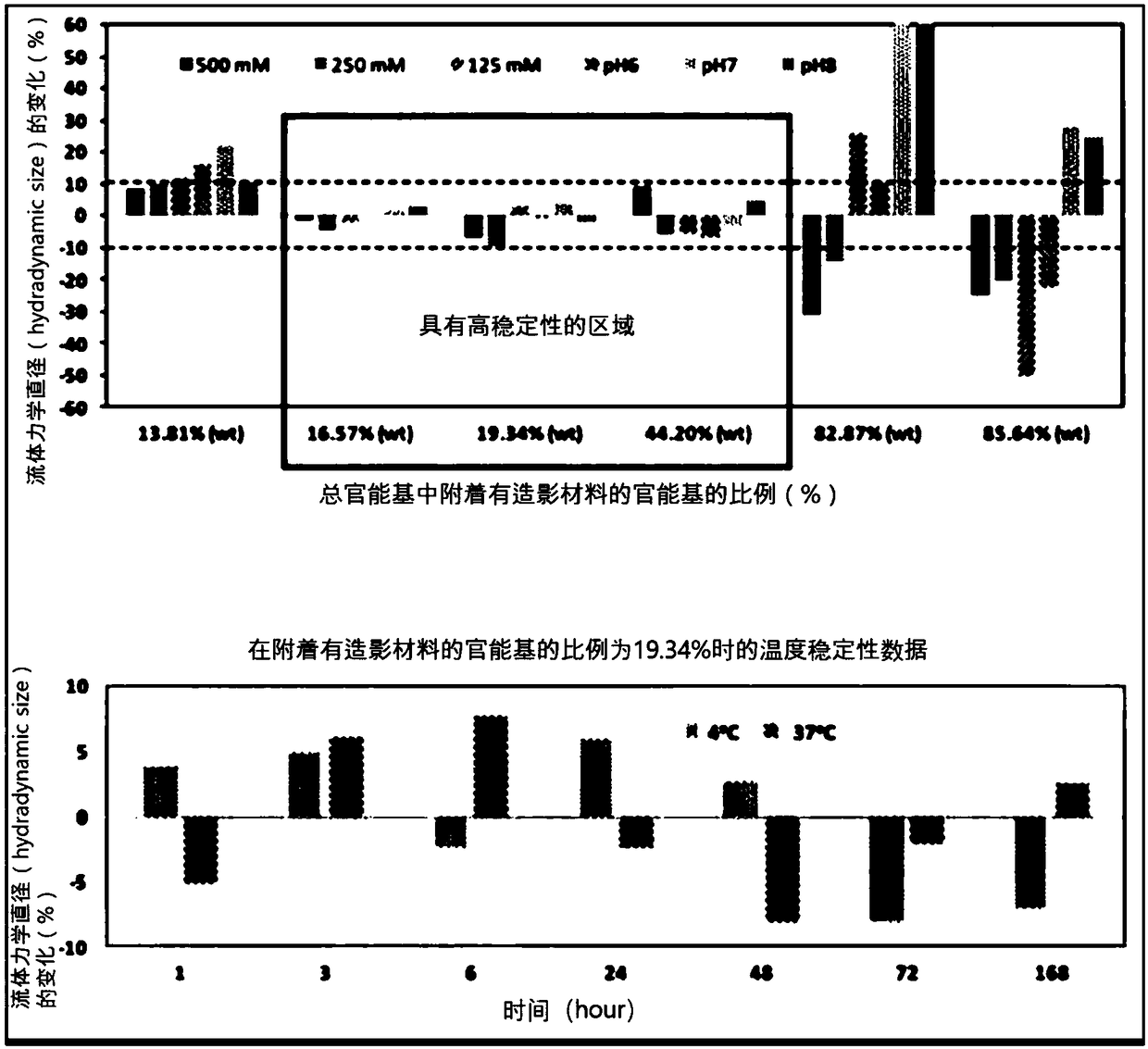
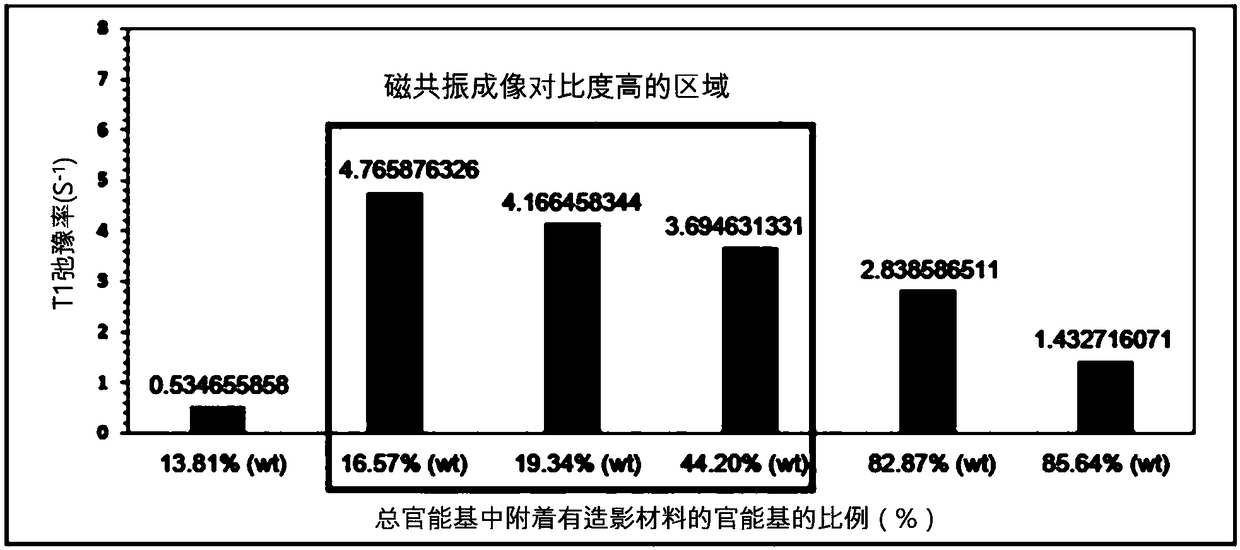
[0105] In the introduction step of the T1 type contrast material in the above-mentioned embodiment 1, when the weight (weight) of the total dextran nanocarrier is 100, the amount of the T1 type contrast material (FeCl2, FeCl3) (based on Fe metal) Adjusted to the following 0.1% (wt), 1% (wt), 2.5% (wt), 5.0% (wt), 10.0% (wt), 25% (wt), 50% (wt) and 100% (wt ), thereby adjusting the percentage of T1-type contrast material attached to the dextran nanocarriers. The hydrodynamic diameters of the synthesized nanoparticle contrast agents were determined to be about 4.7 nm, 4.8 nm, 5.8 nm, 6.5 nm, 7.2 nm, 9.0 nm, 10.0 nm, and 25 nm, respectively, with increasing amounts of T1-type contrast material. Quantification of T1-type contrast material attached to dextran nanocarriers was performed using an inductively coupled plasma mass spectrometer (ICP-MS, PerkinElmer, USA). Sp...
Embodiment 3
[0106] Embodiment 3: the quantification of the hydrophilic functional group on the nanocarrier surface
[0107] Amine (-NH2) functional groups were introduced into the surface of the nanocarriers prepared in Example 1 above, so for the quantification of the hydrophilic functional groups on the surface of the nanocarriers, TNBSA assay, a well-known quantitative method for amines, was used. Specifically, 0.25 mL of 0.01% (w / v) TNBSA (2,4,6-Trinitrobe nzenesulfonic Acid, Thermo, USA) aqueous solution was added to 0.5 mL of nanocarriers at a concentration of 10 mg / mL, and heated at 37° C. After reacting at temperature for 2 hours, 0.25 mL of 10% SDS (sodium dodecyl sulfate, Sigma, USA) aqueous solution and 0.125 mL of 1M HCl (Sigma, USA) were added, and the absorbance was measured at a wavelength of 339 nm. At this time, in order to quantify the amount of amine groups by absorbance, five different concentrations of lysine (Lysine) (Sigma, USA) solutions were measured using TN BSA ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydrodynamic diameter | aaaaa | aaaaa |
| hydrodynamic diameter | aaaaa | aaaaa |
| hydrodynamic diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com