Vcn enhancer compositions and methods of using the same
A technology of promoters and elongation factors, applied in biochemical equipment and methods, drug combinations, and the use of vectors to introduce foreign genetic materials, etc., can solve the problem of difficult and effective transduction of hematopoietic stem cells and progenitor cells, cell toxicity, and increased inefficient transduction Gene therapy costs and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0761] Validation analysis
[0762] Vector copy number (VCN) and transduction efficiency
[0763] Washed cells were resuspended in 2 mL of Stem Cell Growth Medium (SCGM) + cytokines and transferred to standard 12-well non-adherent tissue culture plates. Cells were maintained in a standard humidified tissue culture incubator (5% CO2) for an additional 6 days prior to vector copy number (VCN) analysis or single cell nested PCR to determine transduction efficiency. Both VCN and single-cell nested PCR analysis were performed by qPCR using primers and probes specific for the vector and endogenous control genes. VCN was determined by dividing the amount of vector signal by the amount of endogenous control gene. For single-cell nested PCR analysis, count wells containing cells positive for the endogenous control gene and wells containing labeled cells positive for both the vector and the endogenous control gene, and calculate the proportion of labeled cells or transduction efficien...
example 2
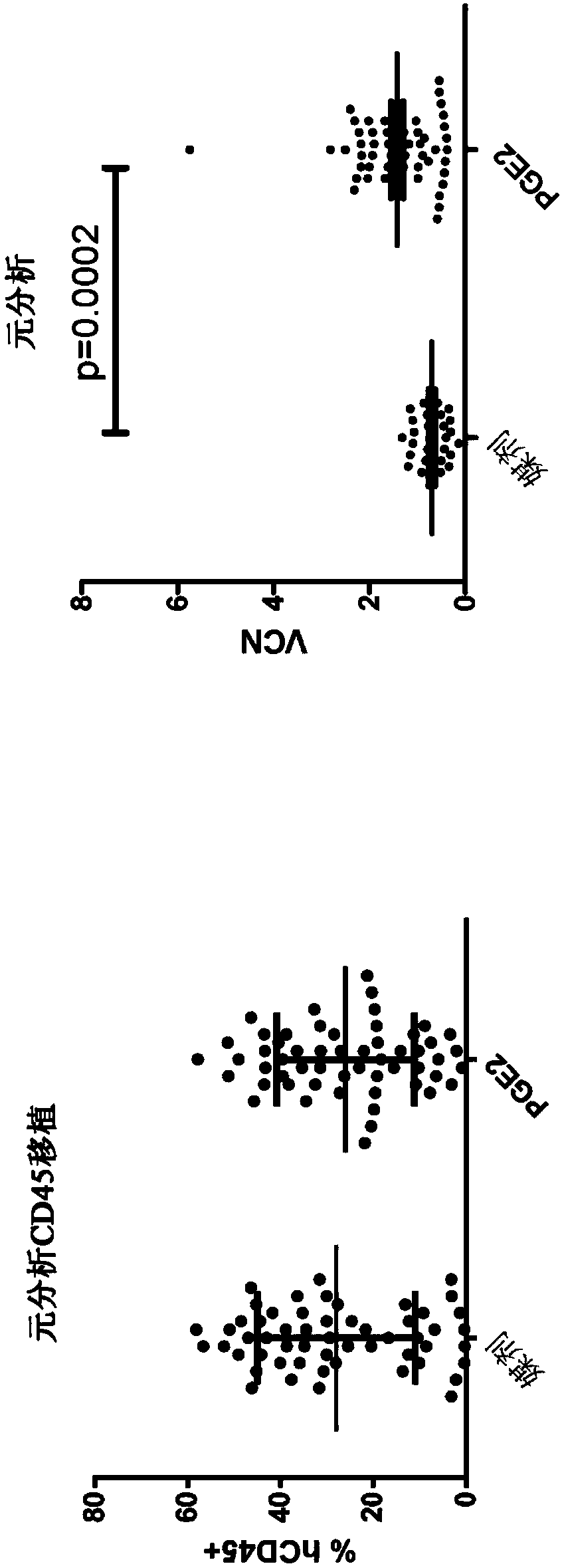
[0771] After PGE 2 processed human CD34 + In vivo analysis of cells
[0772] Contains PGE from healthy donors 2 Meta-analysis of NSG grafts of treated and lentivirally transduced hCD34+ cells (n=3). Human CD34 + Cells were transplanted into submyeloablative female NSG mice and bone marrow (BM) analyzed 4 months after transplantation. BM was harvested from both femurs of NSG mice and half of the samples were stained with anti-hCD45 for approximately 30 min at 4 °C. Following incubation, samples were washed and analyzed for human CD45 by flow analysis using an anti-hCD45 antibody (BD#561864) + cell. In control-treated samples versus PGE-treated 2 hCD45 in mouse BM was not detected between treated samples + The percentages of cells are statistically significantly different. These results indicated that hCD34 + Cellular PGE 2 Treatment did not significantly increase HSC engraftment. figure 1 a. At 4 months after transplantation, the transplanted 2 processed hCD34 +...
example 3
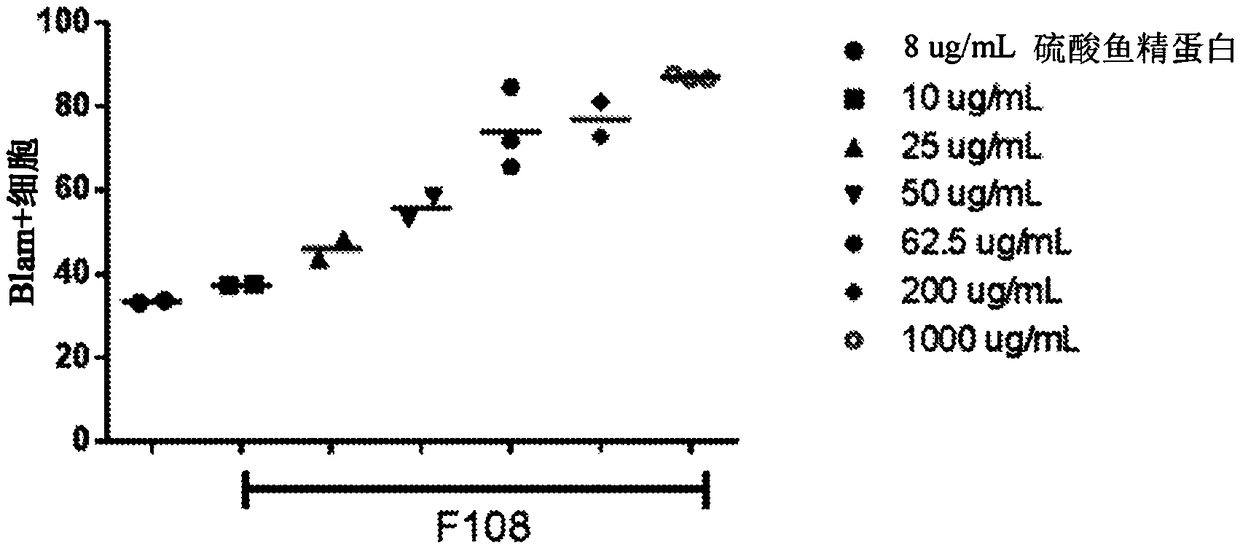
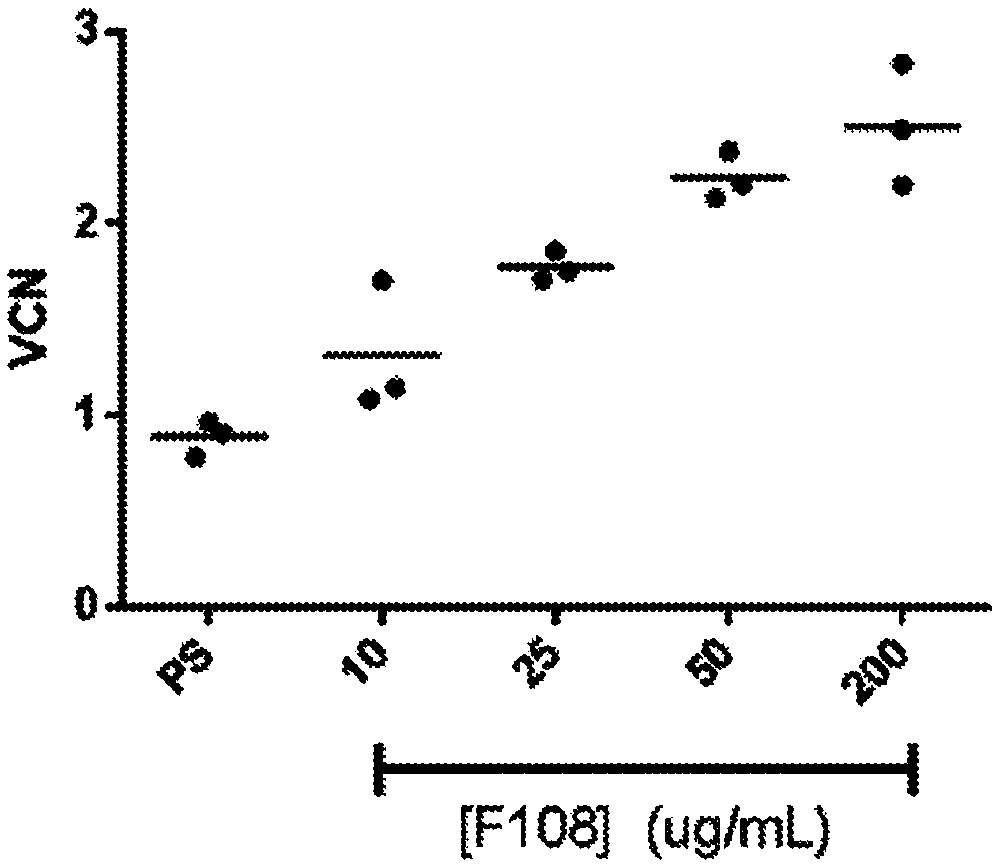
[0774] hCD34+ cells transduced in the presence of increasing concentrations of F108 showed dose-dependent increases in viral entry and VCN
[0775] lentiviral transduction of hCD34 in the presence of protamine sulfate or increasing concentrations of F108 + Cells for 2 hours or 24 hours. Analysis of F108 for lentiviral entry into human CD34 using the BlaM assay + Effects on cells. Cells were transduced with a lentiviral vector encoding a β-lactamase-Vpr fusion protein. After 2 hours of transduction, cells were washed and incubated with a fluorogenic substrate for β-lactamase. Cells were then analyzed by flow cytometry for β-lactamase substrate cleavage, which is associated with lentiviral entry into CD34 + cell related. Flow cytometry analysis revealed that increasing concentrations of F108 during transduction increased LVV entry into hCD34 + cells, an increased percentage of cells with cleaved BlaM substrate was observed. The effect of increased lentiviral entry appeare...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com