Use of LCAT in preparing drug for treating and/or preventing hepatic bone disease
A technology of use and medicine, applied in the field of treatment and prevention of osteoporosis or osteomalacia, to achieve the effect of preventing and treating hepatic bone disease, broadening the field of choice, and relieving osteoporosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
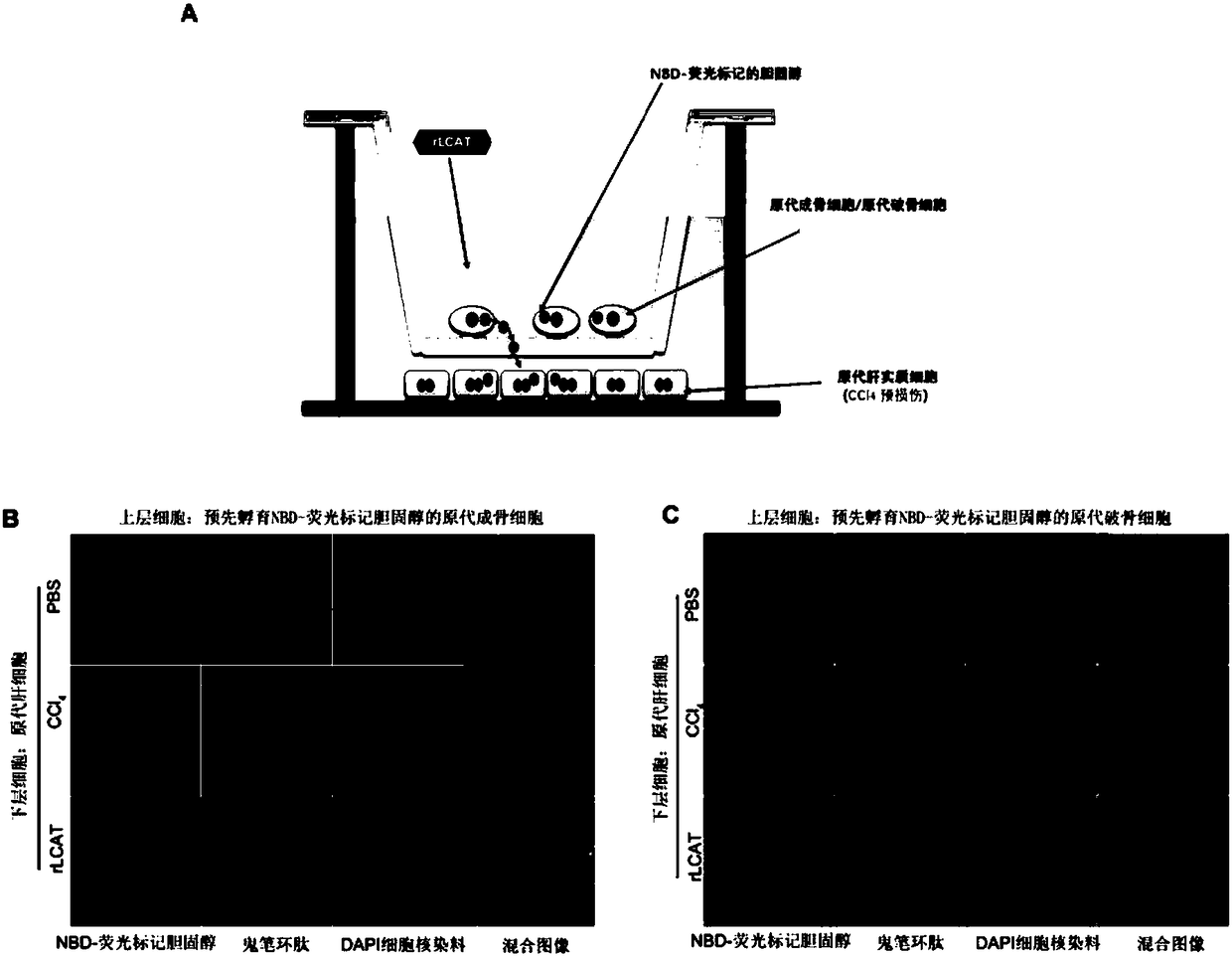
[0024] Example 1. Construction of primary osteoblast / primary osteoclast and primary liver parenchymal cells co-culture system
[0025] Implementation method: Add the isolated primary osteoblasts / primary osteoclasts into a microporous filter culture chamber (MerckMillipore, USA), add NBD fluorescently labeled cholesterol and incubate for 24 hours, then wash off the unphagocytosed cells with PBS solution. NBD fluorescently labeled cholesterol outside primary osteoblasts / primary osteoclasts. The primary hepatic parenchymal cells were transferred to the lower layer of the microporous membrane culture chamber. According to different treatments, the microporous membrane culture chamber system was divided into three groups, namely PBS treatment group, exogenous rLCAT treatment group (100ng / ml), CCl 4 Pre-injury liver parenchymal cell group (1mM), the treatment method is as follows:
[0026] PBS treatment group: PBS solution was added to both the upper layer cells and the lower laye...
Embodiment 2
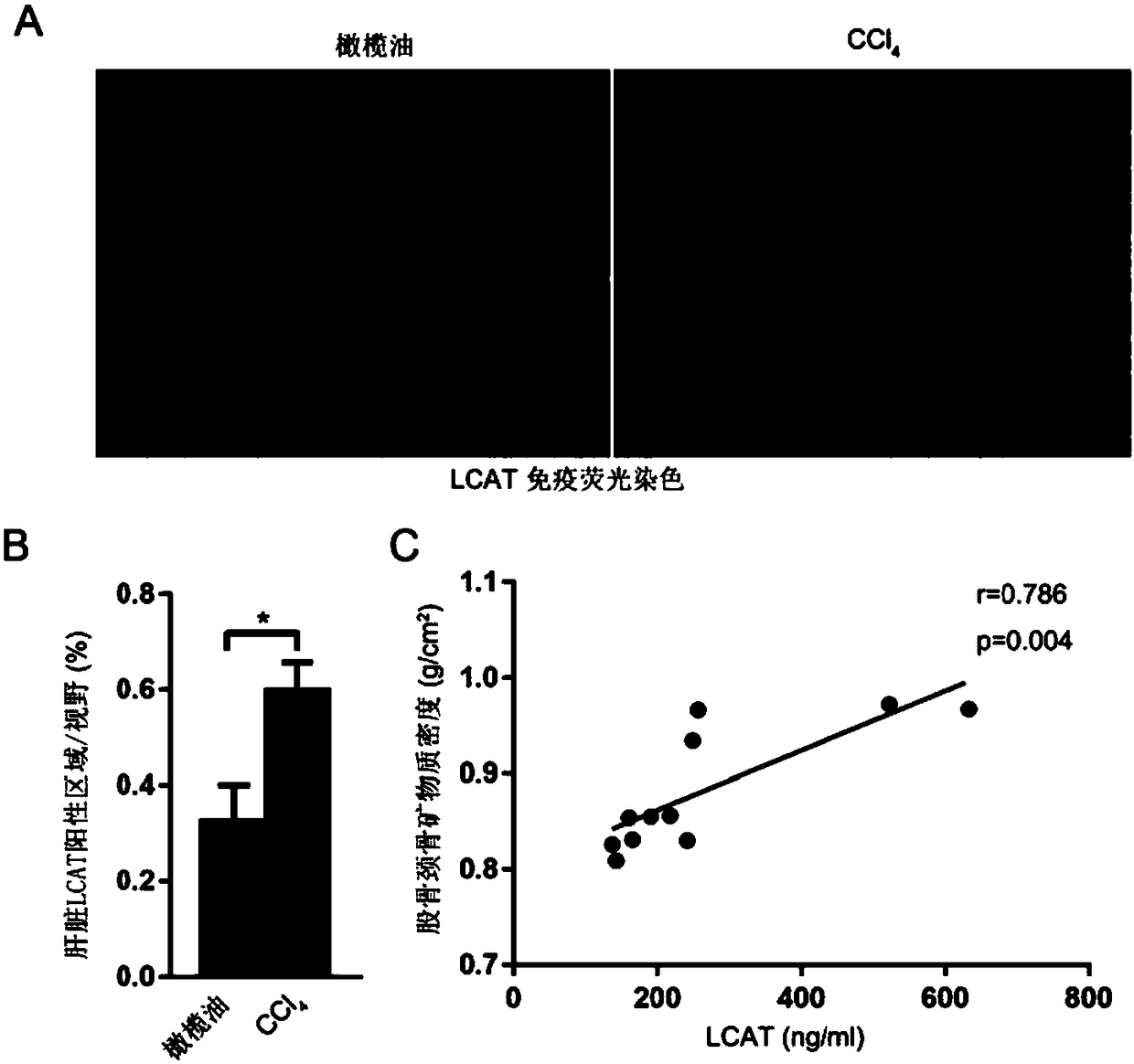
[0031]Example 2. The expression of LCAT in mice with hepatic bone disease and the relationship between the expression of LCAT and bone mineral density in patients with hepatic bone disease
[0032] In order to verify the expression of LCAT in mice with hepatic bone disease and whether there is a certain relationship between the expression of LCAT and bone mineral density in patients with hepatic bone disease, we constructed a mouse model of hepatic bone disease and collected hepatic bone disease Patient serum and measured bone density data.
Embodiment 3
[0035] Example 3. Effect of LCAT on osteoblasts and osteoclasts
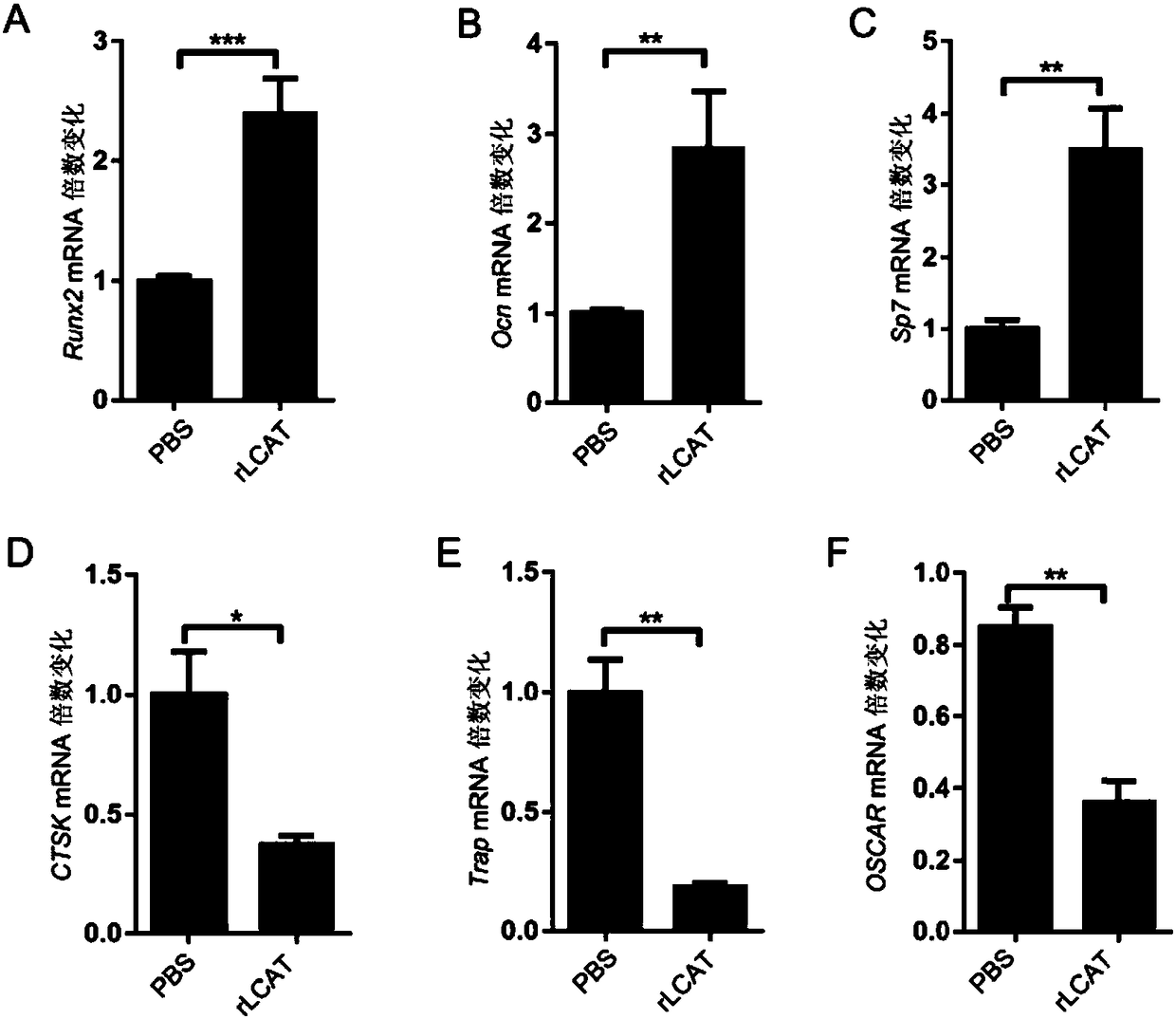
[0036] Implementation method: extract osteoblasts from the skull of 6-day-old male mice (C57BL / 6), extract osteoclast precursors from the bone marrow cavity of the lower limbs of 6-week-old male mice (C57BL / 6), and induce mature osteoclasts with Rank1, Add 200ng / ml exogenous rLCAT solution into the two kinds of cells, and add an equal volume of PBS solution to the control group, incubate the cells in each group for 24 hours, extract RNA, and detect the biological markers of osteoblasts by real-time quantitative PCR Objects Runx2, Ocn and Sp7 ( image 3 A-C in the figure), and osteoclast biomarkers CTSK, Trap and OSCAR ( image 3 D-F graph in ) expression level.
[0037] Example result: as image 3 As shown, after the exogenous rLCAT solution was added to osteoblasts and osteoclasts and incubated for 24 hours, the expression levels of osteoblast-related markers Runx2, Ocn and Sp7 were significantly increased c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com