X-SCID lentivirus carrier, lentivirus as well as preparation method and application thereof
A lentiviral vector and lentiviral technology, applied in the field of genetic engineering, can solve the problems of low immune stimulation and poor safety performance, and achieve the effect of high transfection efficiency, strong stability and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The construction of embodiment 1 lentiviral vector
[0063] This embodiment provides a method for constructing a lentiviral vector, which specifically includes the following steps:
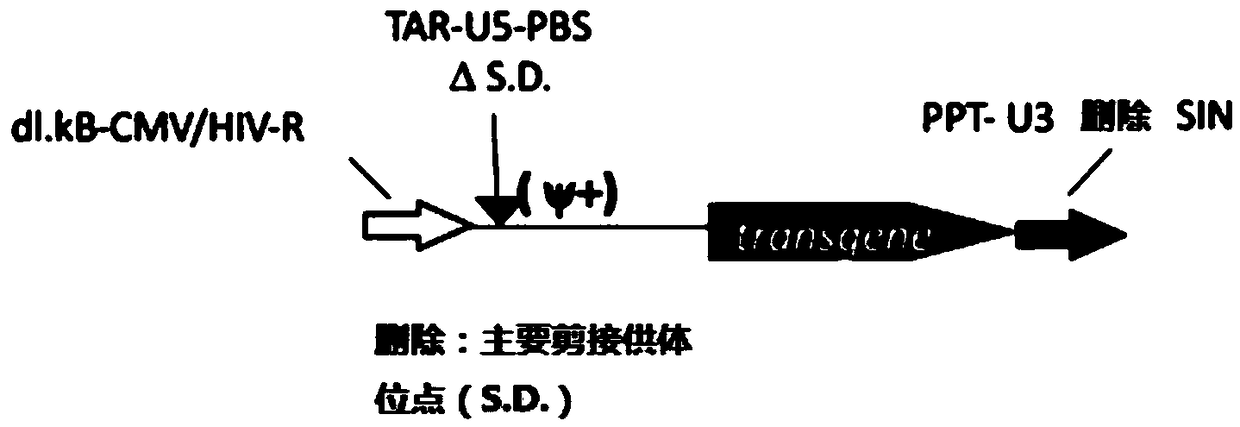
[0064] (1) Schematic diagram of the transformation of the lentiviral vector pTYF as shown in figure 1 As shown, the specific mutation is to mutate the wild-type 5' splice donor site GT to CA, and delete the enhancer in U3. The specific transformation method can be found in "Contributions of Viral Splice Sites and cis-Regulatory Elements to Lentivirus Vector Function, YAN CUI, JOURNAL OF VIROLOGY, July 1999, p.6171–6176”, as follows:
[0065] Transformation of 5' splicing donor site: wild type (SEQ ID NO.3): GGCAAGAGGCGAGGGGCGGCGACTGGTGAGTACGCCAAAAATTTTGACTAGCGGAGGCTA; mutant type (SEQ ID NO.4): GGCAAGAGGCGAGGGGCGGCGACTGCAGAGTACGCCAAAAATTTTGACTAGCGGAGGCTA;
[0066] (2) Insertion of promoter and IL-2RγC gene:
[0067] Synthesize the normal IL-2RγC gene sequence (as shown in SEQ ID NO.1) an...
Embodiment 2
[0072] Preparation and identification of embodiment 2 lentivirus
[0073] 1) Preparation of lentivirus
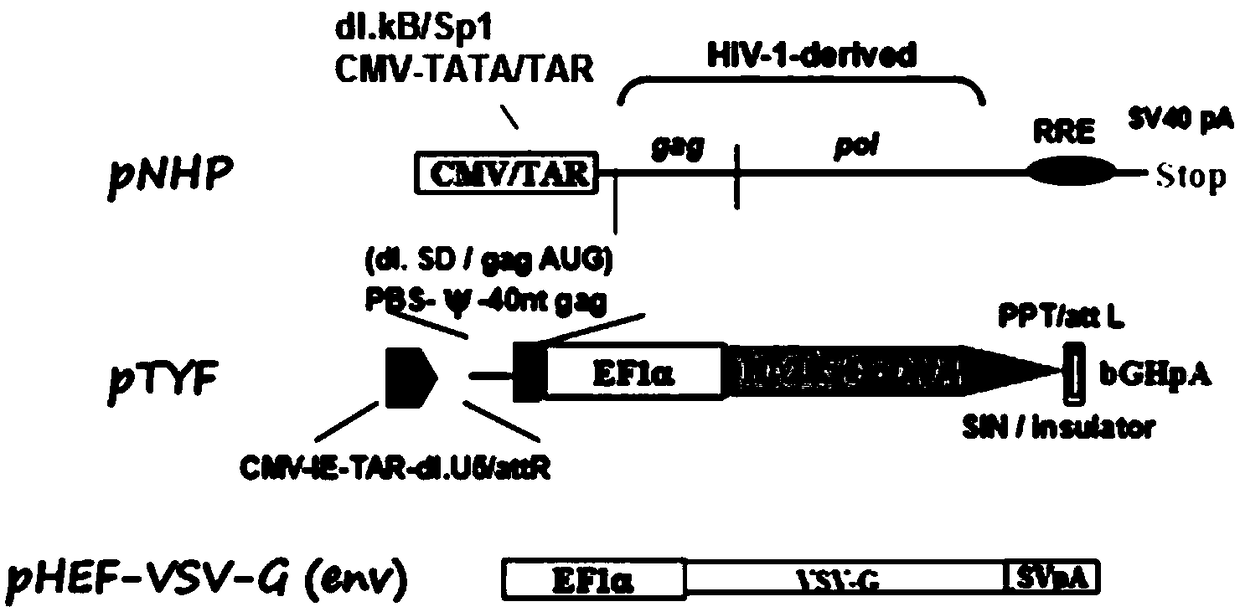
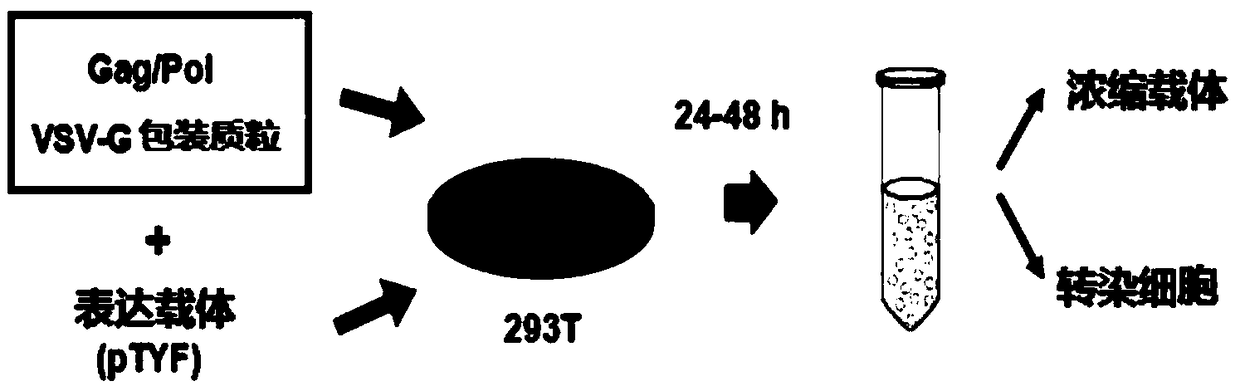
[0074] The lentiviral vector prepared in Example 1 was further packaged, purified and concentrated to obtain the lentivirus. The specific process is as follows: image 3 As shown, the specific steps are as follows:
[0075] (1) Co-transfect the constructed lentiviral vector with the packaging helper plasmids pNHP and pHEF-VSV-G into mammalian cells HEK293T and culture for 24-72h;
[0076] (2) Purifying and concentrating the cultured lentivirus to obtain the lentivirus.
[0077] 2) Identification of lentivirus
[0078] The collected lentivirus-transfected cells carrying normal IL-2RγC gene were identified for protein expression, so as to clarify the expression of IL-2RγC gene in the cells.
[0079] From the results, there is no expression of IL-2RγC protein in the negative control cells without lentivirus transfection, but a significantly larger amount of IL-2RγC protein...
Embodiment 3
[0081] The therapeutic effect of embodiment 3 lentivirus
[0082] The schematic diagram of the treatment process for treating X-SCID disease with the dual stem cell system obtained by transfecting the lentiviral vector of the present invention prepared in Example 2 carrying the normal IL-2RγC gene is as follows Figure 4 As shown, after the patient’s stem cells were mobilized, the peripheral blood of the patient was collected and the hematopoietic stem cells and mesenchymal stem cells were separated, and the double stem cells were transfected with the lentiviral vector carrying the normal IL-2RγC gene to obtain the stem cells carrying the normal IL-2RγC gene. It is injected back into the patient's body by intravenous injection for disease treatment.
[0083] It can be seen from the results that after direct injection of lentivirus, the delivery efficiency and expression level of IL-2RγC gene can be effectively improved.
[0084] In summary, the lentiviral vector of this appli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com