Riboflavin promoting low concentration CO2 electrochemical trapping method
A low-concentration, electrochemical technology, applied in chemical instruments and methods, inorganic chemistry, electrolysis process, etc., can solve the problems of high energy consumption and high capture cost, reduce energy consumption, improve economic feasibility, and reduce capture cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
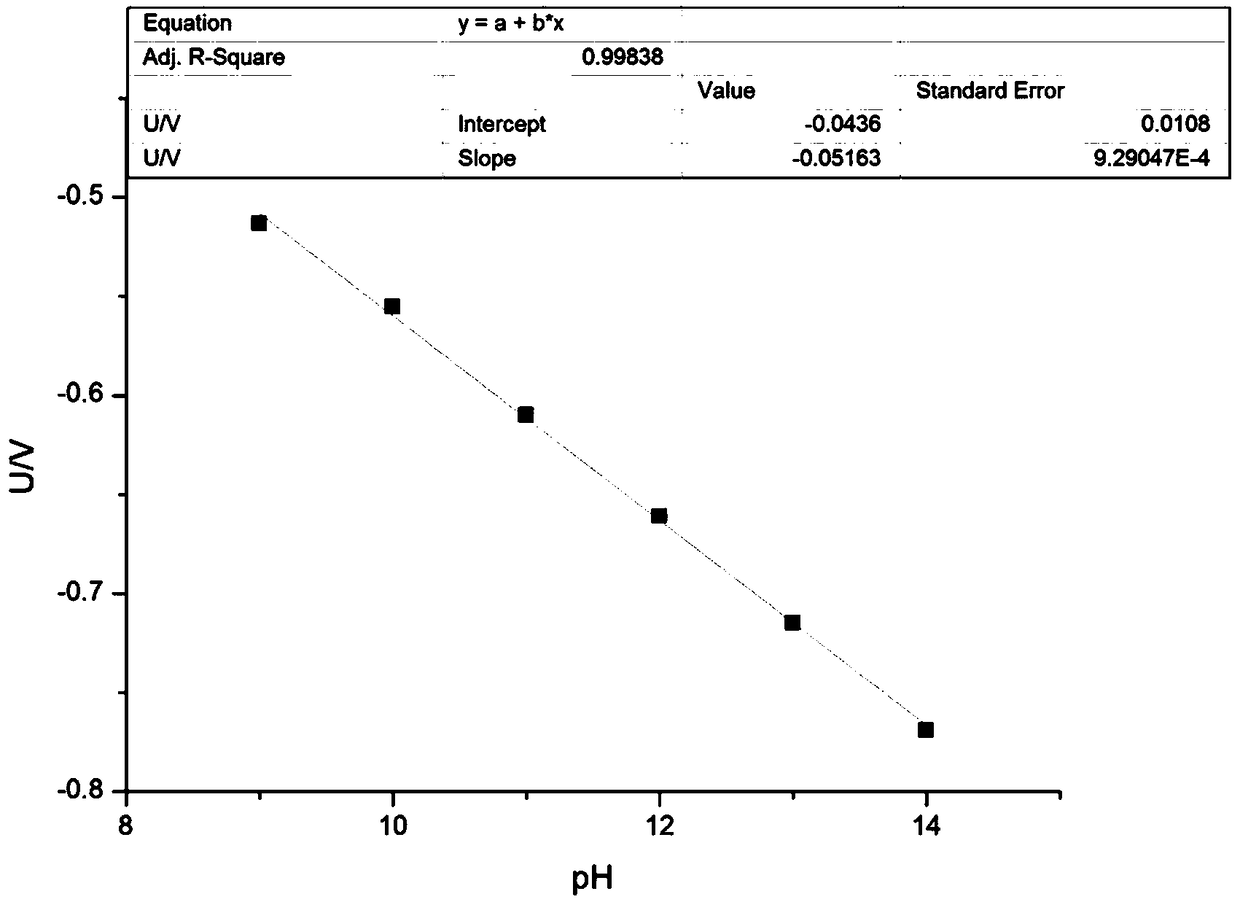
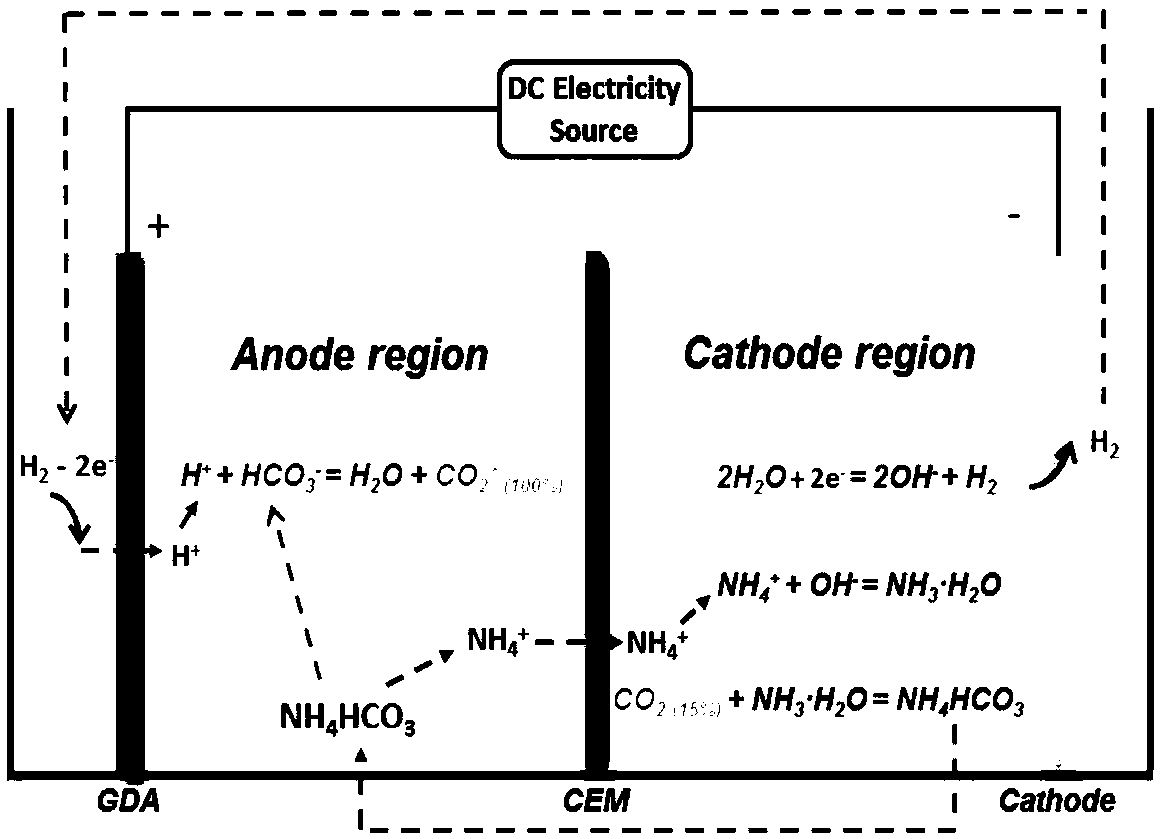
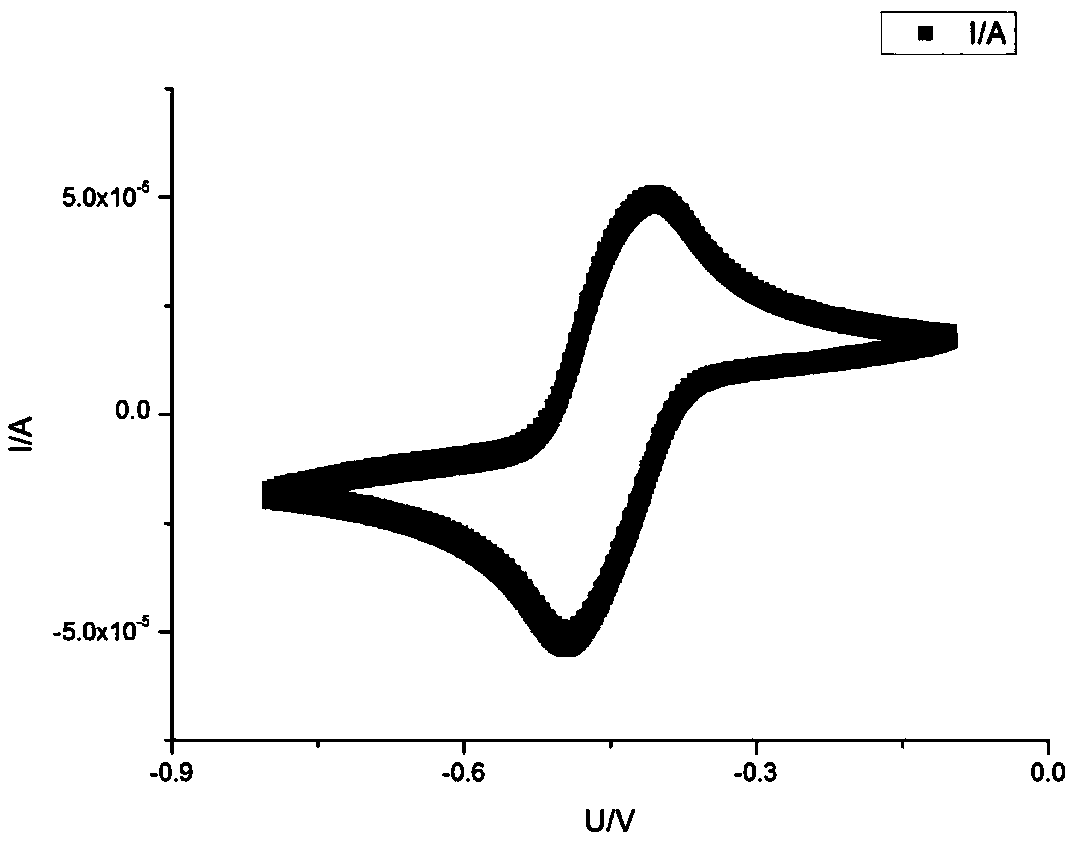
[0060] This example FMN / FMNH 2 Redox pair promotes low CO concentration 2 The principle of electrochemical trapping is as Figure 4 shown.
[0061] in as CO 2 In the shell container of electrochemical collection, a cation exchange membrane that only allows cations to pass through and can prevent anions from passing through is separated into two regions, the anode area and the cathode area.
[0062] Anolyte (1M NaHCO 3 +0.08M FMNH 2 ) and catholyte (1M NaHCO 3 +0.08M FMN) is placed in a 200mL airtight storage tank, and the flow rate of 15mL / min is circulated between the electrolyzer device and the storage tank through a pump, and graphite felt is used as the electrode material, and a DC power supply is applied between the positive and negative electrodes (IT6932A, Itech) power supply, the temperature of the electrolytic cell is 50 ℃.
[0063] Under the action of current, FMN gets two electrons at the cathode, from KHCO 3 The solution receives two protons to form reduced...
example 2
[0070] in as CO 2 In the shell container of electrochemical collection, a cation exchange membrane that only allows cations to pass through and can prevent anions from passing through is separated into two regions, the anode area and the cathode area.
[0071] Anolyte (0.3M NaHCO 3 +0.06M FMNH 2 ) and catholyte (0.3M NaHCO 3 +0.06M FMN) placed in a 200mL airtight storage tank, circulated at a flow rate of 15mL / min between the electrolyzer device and the storage tank through a pump, and 10mL / min of 12% CO was introduced into the cathode area 2 CO 2 / N 2 mixed composition. Graphite felt was used as the electrode material, a DC power supply (IT6932A, Itech) was applied between the cathode and anode electrodes, and the temperature of the electrolytic cell was 55°C.
[0072] When the current density is maintained at 50mA cm -2 When the average voltage is 0.075V, than 10mA cm -2 The 0.065V at the time has increased by 15.3%. From this calculation, every ton of CO captured 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com