Chinese medicinal pill for treating coronary heart disease and preparation method
A traditional Chinese medicine pill and a technology for coronary heart disease, which are applied in the field of traditional Chinese medicine pills for treating coronary heart disease and their preparation, can solve the problems of different drug effects, no effect, affecting the quality of life of patients, etc., so as to increase coronary blood flow and prevent myocardial infarction. , the effect of reducing myocardial oxygen consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] A traditional Chinese medicine pill for treating coronary heart disease, comprising 0.5 parts of red ginseng, 3 parts of astragalus, 6 parts of cassia seed, 2 parts of Radix Ophiopogon japonicus, 0.5 part of agarwood, 3 parts of kudzu root, 10 parts of mulberry root, 1 part of schisandra, and 0.5 part of dandelion 2 parts of Rhizoma Chuanxiong, 10 parts of Salvia miltiorrhiza, 10 parts of honeysuckle, 2 parts of medlar, 0.3 parts of amber, 3 parts of safflower, 3 parts of sealwort, 2 parts of golden cherry, 0.1 part of cinnabar, 5 parts of Maodongqing, Zhishouwu 3 parts, Polygonatum 2 parts, Panax notoginseng 1 part, Chishao 2 parts, Tangerine peel 3 parts, Eucommia 2 parts, Blood 0.5 part, Hawthorn 6 parts, Turmeric 1 part, Cuscuta 2 parts, Shichangpu 2 parts, Licorice 2 parts, 3 parts of angelica, 3 parts of psoralen.
[0061] Its preparation method comprises the following steps:
[0062] (A) Dipping, purifying the ammonium rhenate solution, red ginseng, astragalus, ...
Embodiment 2
[0070] A traditional Chinese medicine pill for treating coronary heart disease, comprising 1 part of red ginseng, 4 parts of astragalus, 7 parts of cassia seed, 3 parts of Ophiopogon japonicus, 1 part of agarwood, 12 parts of kudzu root, 12 parts of mulberry, 1.5 parts of schisandra, 0.8 part of jiangxiang, 3 parts of Chuanxiong, 13 parts of Salvia, 13 parts of honeysuckle, 3 parts of medlar, 0.5 parts of amber, 4 parts of safflower, 5 parts of sealwort, 3 parts of golden cherry, 0.4 part of cinnabar, 6 parts of Maodongqing, 4 parts of Zhiwu , Polygonatum 3 parts, Panax notoginseng 1.5 parts, Chishao 3 parts, Tangerine peel 5 parts, Eucommia 3 parts, Blood 1 part, Hawthorn 7 parts, Turmeric 2 parts, Cuscuta 2.5 parts, Shichangpu 3 parts, Licorice 4 parts , 4 parts of angelica, 3 parts of psoralen.
[0071] Its preparation method comprises the following steps:
[0072] (A) Dipping, purifying the ammonium rhenate solution, red ginseng, astragalus, cassia, radix radix, agarwood,...
Embodiment 3
[0079] A traditional Chinese medicine pill for treating coronary heart disease, comprising 0.8 parts of red ginseng, 3.5 parts of astragalus, 6.5 parts of cassia seed, 2.5 parts of Radix Ophiopogon japonicus, 0.8 part of agarwood, 3.5 parts of kudzu root, 11 parts of mulberry, 1.2 parts of schisandra, and 0.6 2.5 parts of Rhizoma Chuanxiong, 11 parts of Salvia miltiorrhiza, 12 parts of honeysuckle, 2.8 parts of medlar, 0.4 parts of amber, 3.6 parts of safflower, 4.5 parts of sealwort, 2.5 parts of golden cherry, 0.3 part of cinnabar, 5.5 parts of Maodongqing, Zhishouwu 3.4 parts, Polygonatum 2.5 parts, Panax notoginseng 1.2 parts, Radix Paeoniae Rubra 2.3 parts, Tangerine peel 4.2 parts, Eucommia 2.5 parts, Blood 0.8 parts, Hawthorn 6.5 parts, Turmeric 1.5 parts, Cuscuta 2.3 parts, Shichangpu 2.5 parts, Licorice 3 parts, 3.5 parts of angelica, 2.5 parts of psoralen.
[0080] Its preparation method refers to Example 2.
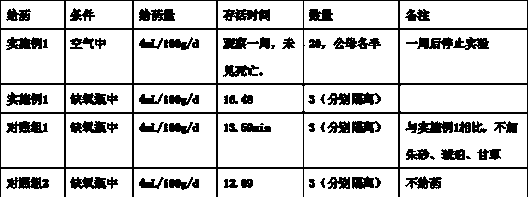
[0081] The inventor has been practicing medicine and tre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com