Preparation method and application of targeted nano delivery system for cerebral ischemia
A nano drug delivery system and cerebral ischemia technology, applied in the field of biomedicine, can solve the problems of poor water solubility, poor permeability, and low bioavailability, and achieve the effects of reducing toxic and side effects, improving efficiency, and solving poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
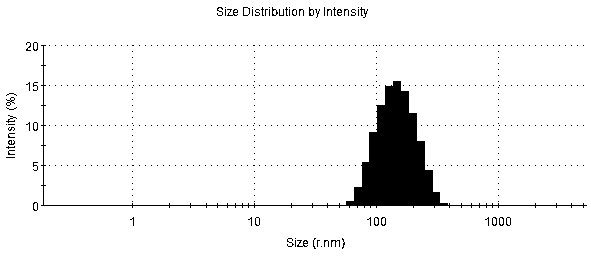
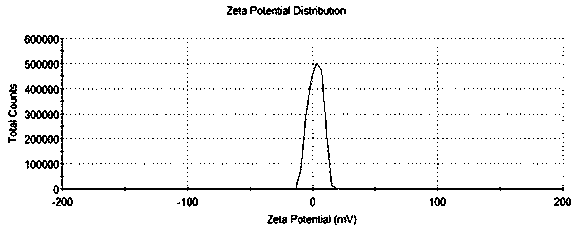
[0035] Weigh 13 mg of 5.0 generation PAMAM dendrimers and 31.0 mg of MAL-PEG-NHS respectively, the molar ratio of the two is 1:10, dissolve in 10 ml of PBS solution with pH 8.38, at room temperature under N 2 Protected from light and stirred for 2 h, the NHS group in the bifunctional polyethylene glycol (MAL-PEG-NHS) reacted specifically with the amino group on the surface of PAMAM, and the reacted solution was transferred to a centrifugal ultrafiltration tube (MW10kDa) , rotate at 8000-10000 rpm / min, centrifuge for 10-15 min, centrifuge 5-8 times, replace the buffer solution with deionized water each time, remove unreacted MAL-PEG-NHS, freeze-dry to obtain PEG-PAMAM, and hydrogen NMR spectroscopy (H 1 -NMR) characterization, such as figure 1 Shown in A.
Embodiment 2
[0037] Weigh 13 mg of 5.0 generation PAMAM dendrimers and 31.0 mg of MAL-PEG-NHS respectively, the molar ratio of the two is 1:10, dissolve in 10 ml of PBS solution with pH 8.38, at room temperature under N 2 Protected from light and stirred for 2 hours, the NHS group reacted specifically with the amino groups on the surface of PAMAM, and the solution after the reaction was transferred to a centrifugal ultrafiltration tube (MW10kDa) at a speed of 8000-10000 rpm / min, and centrifuged for 10-15 minutes. Centrifuge 5-8 times, replace the buffer solution with deionized water each time, remove unreacted MAL-PEG-NHS, and freeze-dry to obtain PEG-PAMAM. According to the ratio of PAMAM to T7 peptide molar ratio of 1:5, respectively Take 1 mgT 7 Peptides were dissolved with 14.5 mg PEG-PAMAM in 2 ml PBS pH 7.00 at room temperature under N 2 Protect the shading and stir the reaction for 24 hours, transfer the reacted solution to a centrifugal ultrafiltration tube (MW10kDa), rotate at 80...
Embodiment 3
[0039] Weigh 13 mg of 5.0 generation PAMAM dendrimers and 37.3 mg of MAL-PEG-NHS respectively, the molar ratio of the two is 1:12, dissolve in 10 ml of PBS solution with pH 8.38, and store under N 2 Stir the reaction for 2 hours under protection and shading. The NHS group reacts specifically with the amino group on the surface of PAMAM. Transfer the reacted solution to a centrifugal ultrafiltration tube (MW10kDa), centrifuge at 8000-10000rpm / min for 10-15 min, and centrifuge for 5 ~8 times, each time the buffer solution was replaced with deionized water to remove unreacted MAL-PEG-NHS, and freeze-dried to obtain PEG-PAMAM. According to the molar ratio of PAMAM and T7 peptide of 1:5, 1 mg of T7 peptide and 14.5 mg of PEG-PAMAM were dissolved in 2 ml of PBS solution with pH 7.00. 2 Protect the shading and stir for 24 hours, transfer the reacted solution to a centrifugal ultrafiltration tube (MW10kDa), centrifuge at 8000-10000 rpm / min for 10-15 min, centrifuge 5-8 times, replace ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com