Method for preparing ethanol by hydrogenating acetic acid
A technology for producing ethanol and acetic acid, which is applied in chemical instruments and methods, preparation of hydroxyl compounds, and preparation of organic compounds. It can solve the problems of small specific surface area and poor catalytic performance of transition metal phosphides, and achieve good catalyst activity and stability. Small impact on the environment, avoiding the effects of post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
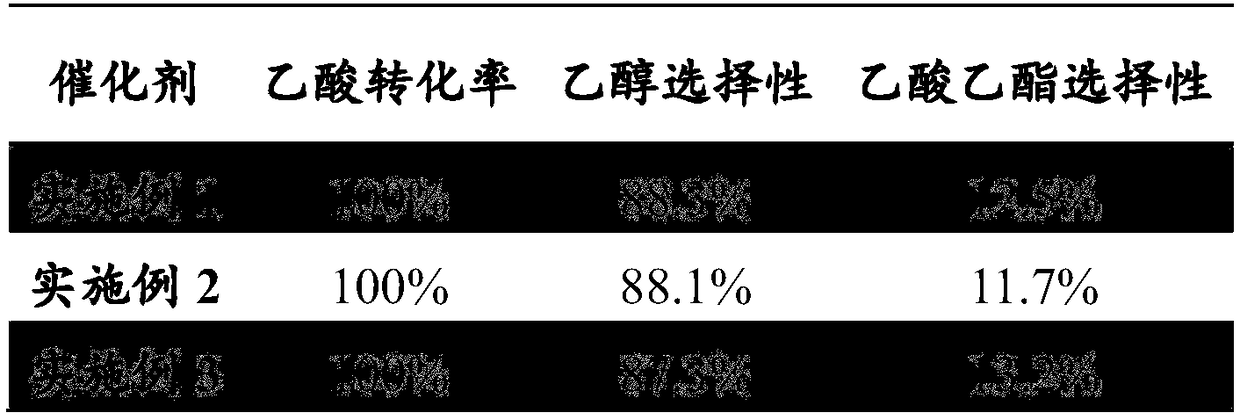
Embodiment 1
[0028] Catalyst preparation
[0029] S1. Weigh a certain amount of carbon nanotubes with an outer diameter of 2nm, and add HNO with a mass fraction of 35% 3 solution and 20ml mass fraction of 15% H 2 o 2 The solution was heated and refluxed at 100°C for 2 to 3 hours, taken out, cooled to room temperature, washed, filtered and dried by conventional methods to obtain surface-modified carbon nanotubes;
[0030] S2. Using the modified carbon nanotubes prepared above as a carrier, Ni(H2PO2)2.6H2O nickel source, Ni(H2PO2)2.6H2O and NaH2PO2.H2O phosphorus source to prepare a supported catalyst. Weigh 10g of porous silica gel, prepare 12mL impregnation solution from 4.0g (1.3478×10-2mol) of Ni(H2PO2)2·6H2O, 1.43g (1.3492×10-2mol) of NaH2PO2·H2O and distilled water, the nickel and phosphorus The concentrations are 1.1232×10-3mol / mL and 3.3707×10-3mol / mL respectively. The volume of the impregnating solution is equal to the volume of the maximum adsorbed water of the catalyst carrier. T...
Embodiment 2
[0032] Catalyst preparation
[0033] S1. Weigh a certain amount of carbon nanotubes with an outer diameter of 2nm, and add HNO with a mass fraction of 35% 3 solution and 20ml mass fraction of 15% H 2 o 2 The solution was heated and refluxed at 100°C for 2 to 3 hours, taken out, cooled to room temperature, washed, suction filtered and dried by conventional methods to obtain surface-modified carbon nanotubes.
[0034] S2. Using the carbon nanotubes prepared above as a carrier, Ni(H2PO2)2.6H2O nickel source, Ni(H2PO2)2.6H2O and NaH2PO2.H2O phosphorus source to prepare a supported catalyst. Weigh 10g of porous silica gel, prepare 36mL impregnation solution from 4.0g (1.3478×10-2mol) of Ni(H2PO2)2·6H2O, 1.43g (1.3492×10-2mol) of NaH2PO2·H2O and distilled water, the nickel and phosphorus The concentrations are 3.7439×10-4mol / mL and 1.1236×10-3mol / mL respectively, the volume of the impregnation solution is three times the maximum volume of water absorbed by the catalyst carrier, a...
Embodiment 3
[0036] Catalyst preparation
[0037] S1. Weigh a certain amount of carbon nanotubes with an outer diameter of 2nm, and add HNO with a mass fraction of 35% 3 solution and 20ml mass fraction of 15% H 2 o 2 The solution was heated and refluxed at 100°C for 2 to 3 hours, taken out, cooled to room temperature, washed, suction filtered and dried by conventional methods to obtain surface-modified carbon nanotubes.
[0038] S2. Using the carbon nanotubes prepared above as a carrier, Ni(H2PO2)2.6H2O nickel source, Ni(H2PO2)2.6H2O and NaH2PO2.H2O phosphorus source to prepare a supported catalyst. Weigh 10g of porous silica gel, prepare 12mL impregnation solution from 4.0g (1.3478×10-2mol) of Ni(H2PO2)2·6H2O, 1.43g (1.3492×10-2mol) of NaH2PO2·H2O and distilled water, the nickel and phosphorus The concentrations are 1.1232×10-3mol / mL and 3.3707×10-3mol / mL respectively. The volume of the impregnating solution is equal to the volume of the maximum adsorbed water of the catalyst carrier. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com