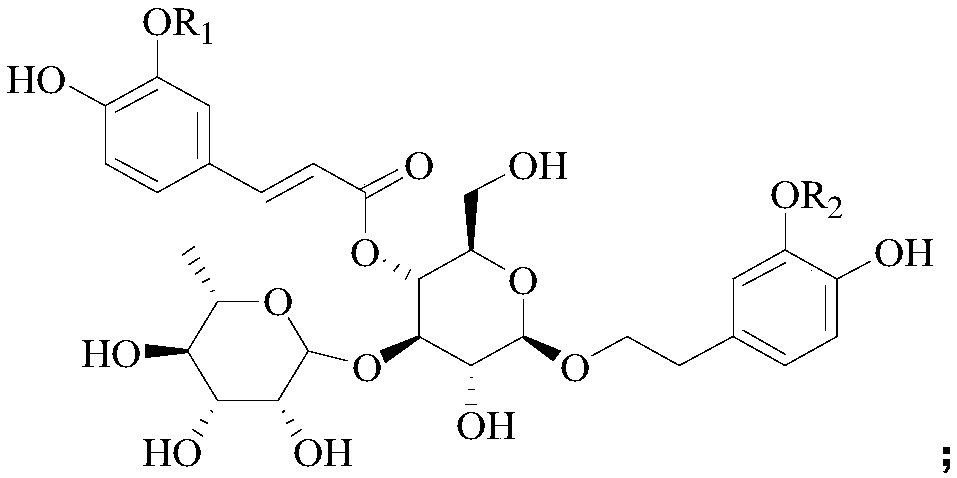
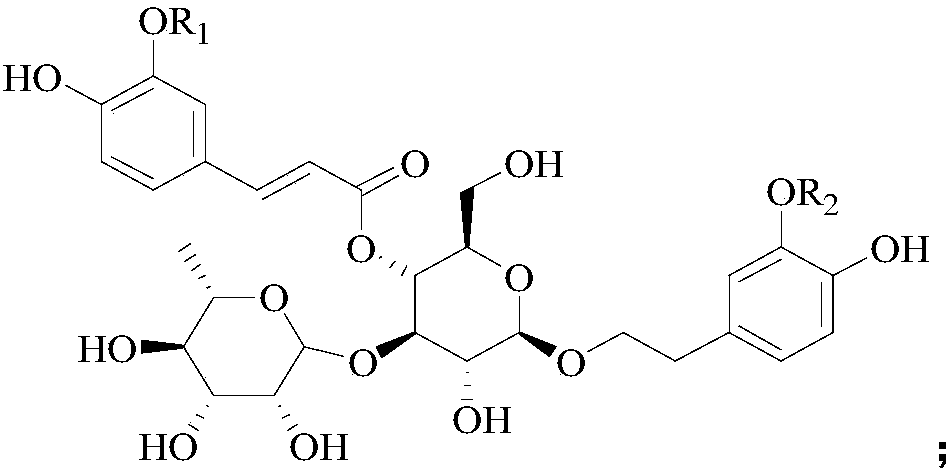
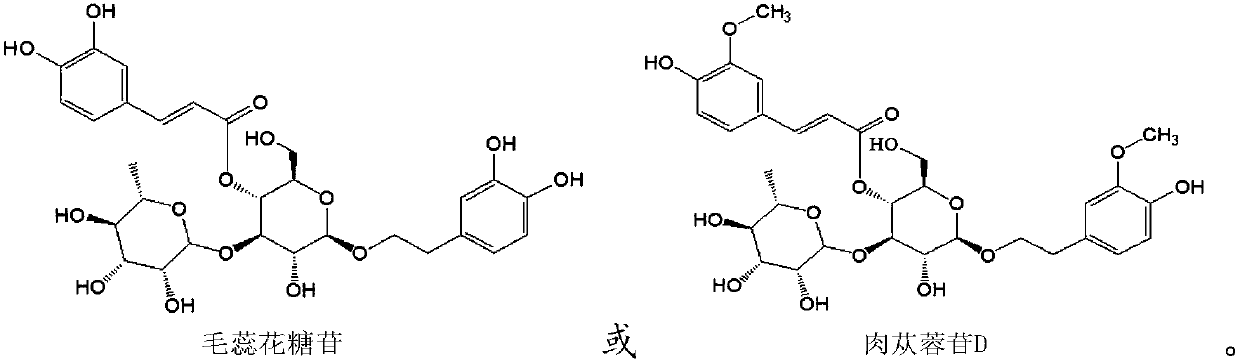
Application of phenylpropanoid glycosides in preparation of IDO (indoleamine-2,3-dioxygenase) inhibitors
A technology of phenylpropanoid glycosides and compounds, applied in the direction of drug combinations, medical preparations containing active ingredients, antibacterial drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of Verascoside and Cistanche glycoside D
[0033] Take the dried succulent stems of Cistanche deserticola Ma, add 10 times the weight of the 75% ethanol aqueous solution by heating and reflux for extraction twice, the first extraction is 2 hours, the second extraction is 1 hour, the extracts are combined, and the Press and concentrate to obtain Cistanche deserticola extract;
[0034] Suspended the Cistanche deserticola extract in 1 times the weight of water, then extracted twice with n-butanol as the extractant, collected the organic phase of the extract, and concentrated under reduced pressure to obtain the extract of the n-butanol extraction part of Cistanche deserticola;
[0035] The n-butanol extract of Cistanche deserticola was purified by AB-8 macroporous resin column chromatography (the diameter of the macroporous resin column was 8 cm, and the column volume was 3.5 L), using water as mobile phase A and ethanol as mobile phase B , and carr...
experiment example 1
[0039] Experimental example 1 Inhibitory Activity of Verbasin and Cistanche D on IDO
[0040] 1. The purpose of the experiment
[0041] HEK293 cells were transfected with plasmid pcDNA3.1-IDO to make it highly express IDO, and then the inhibitory activities of verbascoside and cistanoside D on IDO at the cellular level were determined respectively.
[0042] 2. Experimental method
[0043] HEK 293 cells were seeded in a 96-well plate at a density of 2.5×104 cells / well, cultured in DMEM medium (containing 10% fetal bovine serum, 50U / mL penicillin and 50mg / mL streptomycin), placed at 37°C, humidity 95%, 5% CO 2 cultured in an incubator. After culturing for 24 hours, liposome Lipofectamin 2000 was used to mediate pcDNA3.1-hIDO plasmid transfection, and they were divided into positive control group and experimental group 1-2. The positive control group used 1-methyltryptophan (1-MT) as the test product, and the experimental groups 1-2 used the verbascoside and cistanoside D p...
experiment example 2
[0053] Experimental example 2 Therapeutic effect of phenylpropanoid glycosides on ankylosing spondylitis
[0054] 1. The purpose of the experiment
[0055] The mouse model of ankylosing spondylitis was established by proteoglycan immunization, and then the verascoside and Cistanche D were administered orally. The serum levels of inflammatory markers TNF-α and NF-κB receptor activator ligand (RANKL) were detected by ELISA. , and detected serum IDO activity (Kyn / Trp) to verify the efficacy of phenylpropanoid glycosides for ankylosing spondylitis.
[0056] 2. Experimental method
[0057] 2.1 Experimental animals
[0058] Thirty-two healthy male BALB / c mice, weighing (18±2) g, aged 4-5 weeks, were purchased from Shanghai Slack.
[0059] 2.2 Test drugs
[0060] Verbasin and Cistanche glycoside D prepared in Example 1 were used as test drugs.
[0061] 2.3 Experiment grouping and modeling
[0062] After 1 week of adaptive feeding of mice, 8 of them were taken as blank control...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com