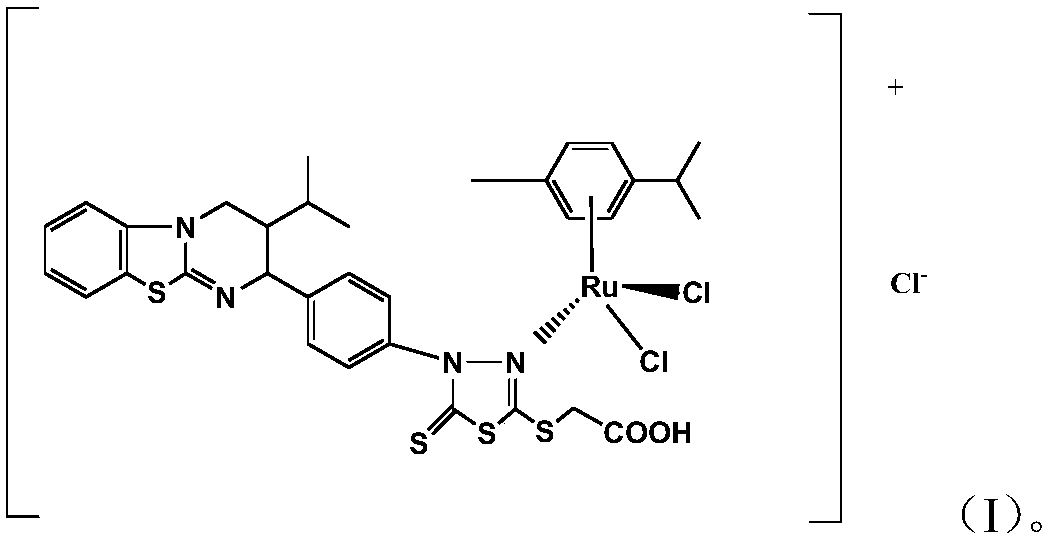
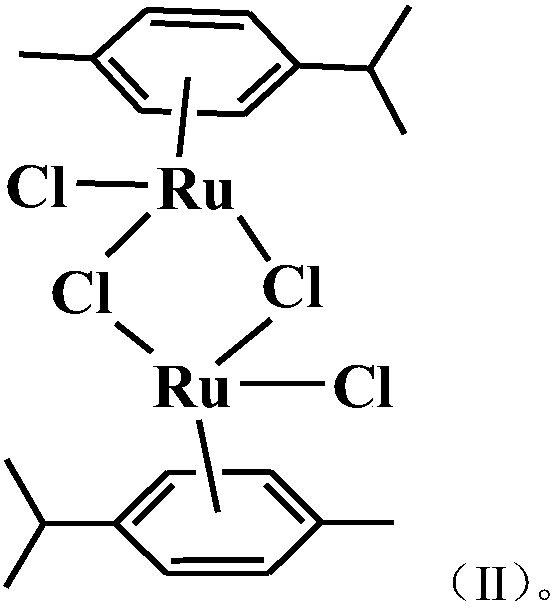
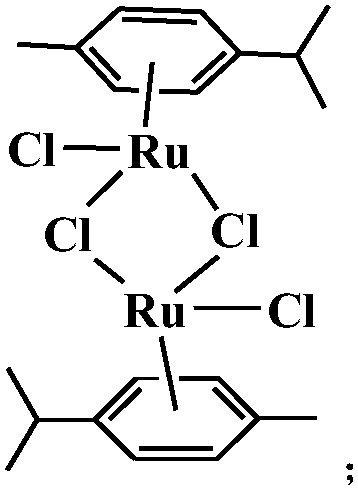
Aryl ruthenium complex with antitumor activity as well as preparation method and application of aryl ruthenium complex
A technology of anti-tumor activity and aryl ruthenium, which is applied to medical preparations of non-active ingredients, anti-tumor drugs, pharmaceutical formulations, etc., to achieve the effects of reducing raw material loss, simple preparation method, and good biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] 1) Preparation of small polypeptide molecules: select amino acid-king resin as the carrier (resin), fully swell the resin with dichloromethane, wash several times with dimethylformamide, and use DBLK (volume ratio piperidine:DMF=1: 4), remove the Fmoc-protecting group (Fmoc is 9-fluorenylmethoxycarbonyl, which can be used as an amino protecting group), then wash several times with dimethylformamide, wash away DBLK, and weigh a suitable condensing agent Benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate and the activator methylmorpholine and the second Fmoc-protected amino acid at the C-terminus (Fmoc-Pro-OH) were used for coupling The connection is detected by the ninhydrin detection method to ensure that the connection is relatively complete, washed several times with dimethylformamide to wash away the remaining residues, activators and condensation agents, coupled according to the amino acid sequence, and all the After the amino acid connection is complete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com