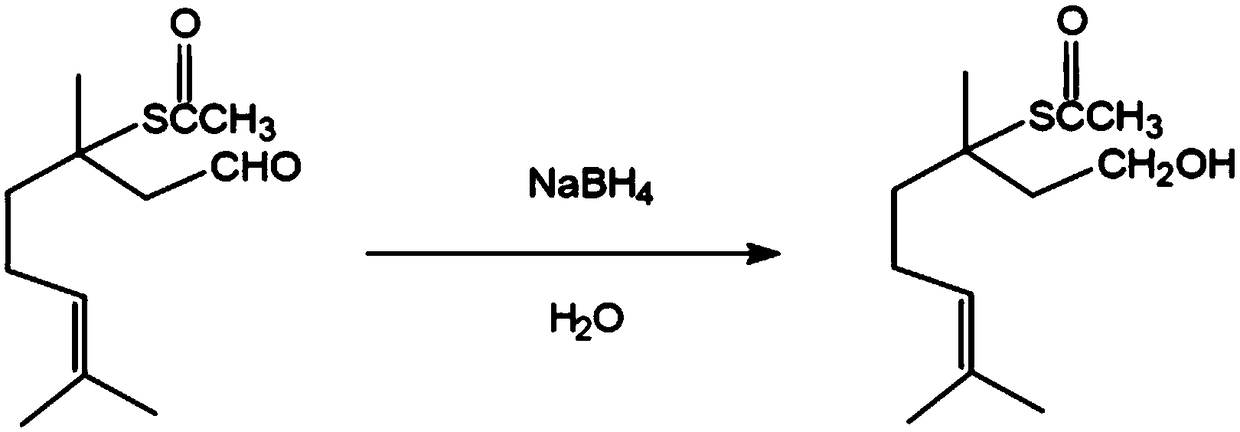
Preparation method of 3,7-dimethyl-3-acetylthio-6-octenol
A technology of acetylthio and dimethyl, applied in the field of preparation of 3,7-dimethyl-3-acetylthio-6-octenol, which can solve the problem of harsh reaction conditions, unfriendly environment and many by-products and other problems, to achieve the effect of short reaction time, low price and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A preparation method of 3,7-dimethyl-3-acetylthio-6-octenol, comprising the following steps:
[0028] (1), at room temperature, 2.02 grams (98.90%, 8.76mmol) 3,7-dimethyl-3-acetylthio-6-octenal, 0.33 grams (8.76mmol) sodium borohydride and 20mL water The mixed solution A formed was stirred and reacted for 5 hours, and the resulting reaction solution was washed with a 10% HCl aqueous solution by mass percentage, and the pH of the washing was neutral;
[0029] (2), step (1) pH washing to neutral reaction solution is extracted with ether, and the organic layer of gained is washed with anhydrous MgSO 4 Dry and filter with filter paper the next day, and the resulting filtrate is evaporated and concentrated by a rotary evaporator to obtain 1.78 g of crude product 3,7-dimethyl-3-acetylthio-6-octenol, which is detected by gas chromatography. The purity is 96.80%, and the yield is 85.30%;
[0030] (3), the crude product of 3,7-dimethyl-3-acetylthio-6-octenol obtained in step (...
Embodiment 2
[0034] A preparation method of 3,7-dimethyl-3-acetylthio-6-octenol, comprising the following steps:
[0035] (1), at room temperature, mix 8.20 grams (98.90%, 35.56mmol) 3,7-dimethyl-3-acetylthio-6-octenal, 1.07 grams (28.30mmol) sodium borohydride and 20mL water The mixed solution A formed was stirred and reacted for 3 hours, and the resulting reaction solution was washed with an aqueous HCl solution with a mass percent concentration of 10%, until the pH of the washing was neutral;
[0036] (2), step (1) pH washing to neutral reaction solution is extracted with ether, and the organic layer of gained is washed with anhydrous MgSO 4 Dry and filter with filter paper the next day, and the resulting filtrate is evaporated and concentrated by a rotary evaporator to obtain 6.79 g of crude product 3,7-dimethyl-3-acetylthio-6-octenol, which is detected by gas chromatography. The purity is 97.00%, and the yield is 80.52%;
[0037] (3), the crude product of 3,7-dimethyl-3-acetylthio-6-o...
Embodiment 3
[0039] A preparation method of 3,7-dimethyl-3-acetylthio-6-octenol, comprising the following steps:
[0040] (1), at room temperature, mix 8.15 grams (98.90%, 35.35mmol) 3,7-dimethyl-3-acetylthio-6-octenal, 0.67 grams (17.72mmol) sodium borohydride and 30mL water The mixed solution A formed was stirred and reacted for 2.5 hours, and the resulting reaction solution was washed with a 10% HCl aqueous solution by mass percentage, and the pH of the washing was neutral;
[0041] (2), step (1) washes the reaction liquid of pH to neutrality and extracts with ether, and the organic layer of gained is washed with anhydrous MgSO 4 Dry and filter with filter paper the next day, and the obtained filtrate is evaporated and concentrated by a rotary evaporator to obtain 6.43g of crude product of 3,7-dimethyl-3-acetylthio-6-octenol, which is detected by gas chromatography. is 96.38%, and the yield is 76.23%;
[0042] (3), the crude product of 3,7-dimethyl-3-acetylthio-6-octenol obtained in s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com