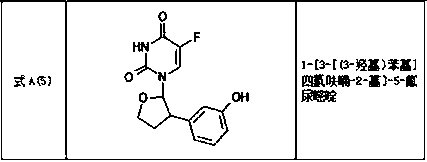
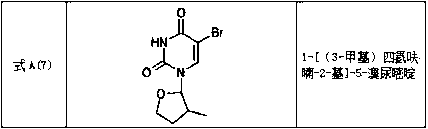
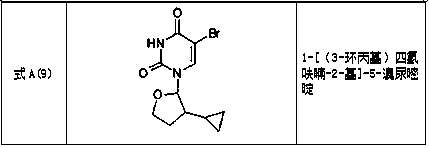
Tetrahydrofuran substituted uracil compound for treating liver cancer as well as pharmaceutical composition and application thereof
A technology for the treatment of liver cancer with tetrahydrofuran, applied in the field of medicine, can solve the problem of prolongation of less than 3 months
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Synthesis of 1-[(3-methyl)tetrahydrofuran-2-yl]-5-fluorouracil.
[0064]
[0065] The synthesis steps are:
[0066] ;
[0067] First, methanol (550g) was placed in a low-temperature constant temperature reaction bath, and hydrogen chloride gas was added to increase the weight to (600g) to stop ventilation, and a methanol solution of hydrochloric acid was prepared as an acidifying agent;
[0068] Next, add 2-methoxy-5-fluorouracil (compound B) (45g) into the solution containing the acidifier and stir, heat it in a water bath to 35°C and keep it warm for 6 hours. The HPLC monitors the end of the reaction and cools down after the end of the reaction. to 15°C, and suction filtered to obtain 5-fluorouracil (compound C) (40.57 g). After suction filtration, the solution was concentrated to recover methanol;
[0069] Finally, under nitrogen protection, 5-fluorouracil (compound C) (40.57g) was added to 3-methyltetrahydrofuran (compound D) (42g), carbon tetrabromide (80ml)...
Embodiment 2
[0071] Synthesis of 1-[[3-(1-chloroisopropyl-2-yl)]tetrahydrofuran-2-yl]-5-fluorouracil.
[0072]
[0073] The synthesis steps are:
[0074] ;
[0075] First, methanol (600g) was placed in a low-temperature constant temperature reaction bath, and hydrogen chloride gas was introduced to increase the weight to (650g) to stop ventilation, and a methanol solution of hydrochloric acid was prepared as an acidifying agent;
[0076] Next, add 2-methoxy-5-fluorouracil (Compound B) (50g) into the solution containing the acidifier and stir, heat it in a water bath to 30°C and keep it warm for 5 hours. HPLC monitors the end of the reaction, and cools down after the end of the reaction To 15°C, filter with suction to obtain 5-fluorouracil (compound C) (46.78g), and concentrate the solution to recover methanol after suction filtration;
[0077] Finally, under nitrogen protection, 5-fluorouracil (compound C) (46.78g) was added to 3-(1-chloroisopropyl) tetrahydrofuran (compound D) (48....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com