Quinoline compound for combined treatment of chondrosarcoma
A technology of chondrosarcoma and compounds, applied in the field of pharmaceutical preparations and medicine, can solve the problems of insensitivity, poor lymphatic circulation, and no chemotherapy regimen for chondrosarcoma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
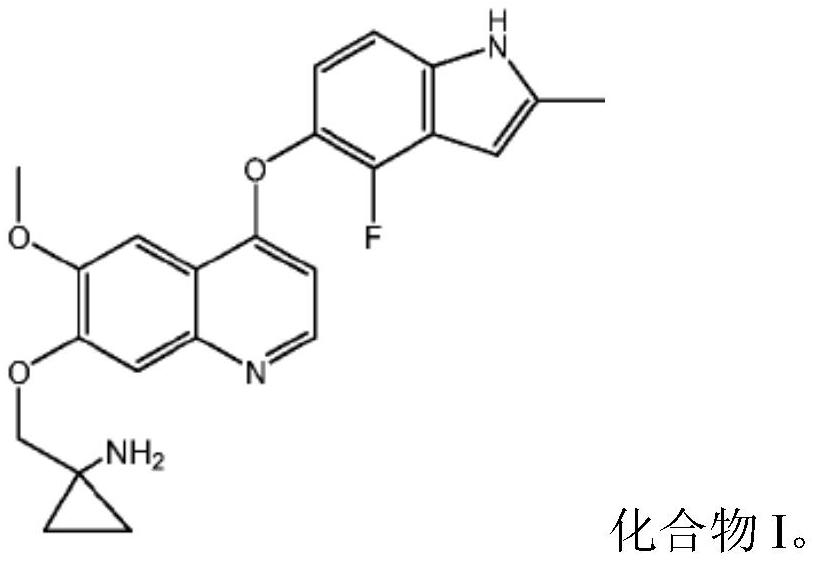
[0058] Example 1 1-[[[4-(4-Fluoro-2-methyl-1H-indol-5-yl)oxy-6-methoxyquinolin-7-yl]oxy]methyl] Cyclopropylamine dihydrochloride
[0059]
[0060] 1-[[[4-(4-Fluoro-2-methyl-1H-indol-5-yl)oxy-6-methoxyquinolin-7-yl was prepared with reference to the method of Example 24 in WO2008112407 ]oxy]methyl]cyclopropylamine, and then according to the preparation method of "Example in salt form" in the specification of WO2008112407, the title compound was prepared.
[0061] Or prepared by referring to the method disclosed in Chinese patent application CN102344438A.
Embodiment 21
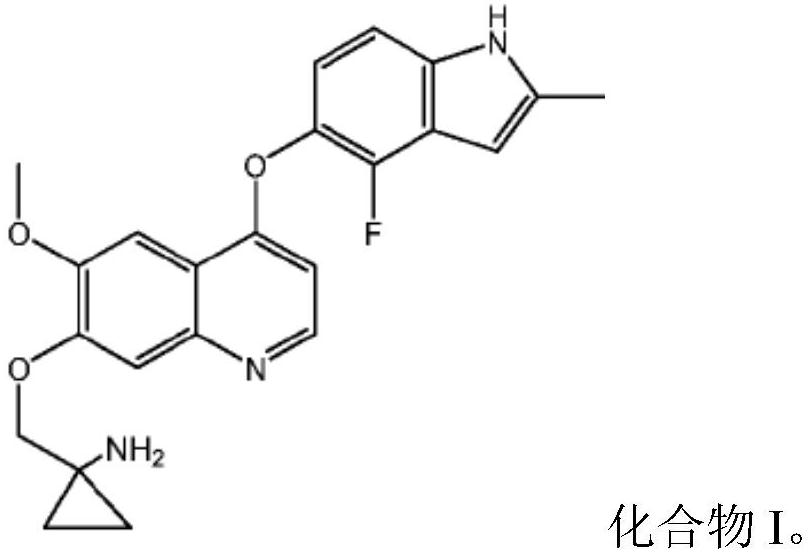
[0062] Example 2 1-[[[4-(4-Fluoro-2-methyl-1H-indol-5-yl)oxy-6-methoxyquinolin-7-yl]oxy]methyl] Preparation of capsules of cyclopropylamine dihydrochloride (dihydrochloride of compound I)
[0063]
[0064] Pulverize the dihydrochloride of compound I, pass through an 80 mesh sieve; then mix with mannitol and hydroxypropyl cellulose; then add the microcrystalline cellulose of the recipe quantity, mix well, pass through a 0.8mm sieve; finally add the recipe quantity of magnesium stearate, mix well and fill the capsules.
[0065] For capsules with other contents of the dihydrochloride of compound I, it can be prepared with reference to the same proportion and prescription above.
Embodiment 3
[0066] Example 3 In vitro experiment
[0067] cell line:
[0068] Human chondrosarcoma cell line CAL-78, SW1353 (source: ATCC).
[0069] Tested drugs:
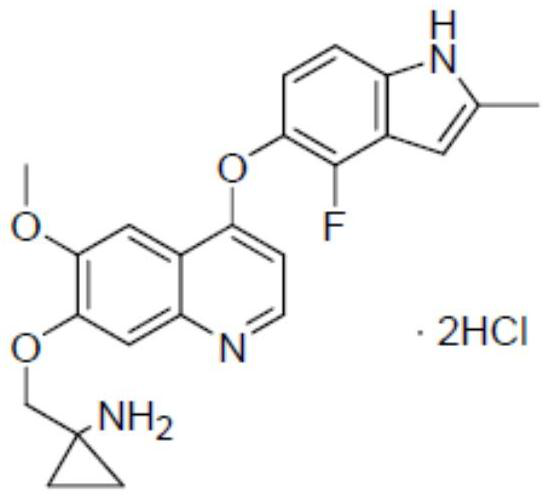
[0070] 1-[[[4-(4-Fluoro-2-methyl-1H-indol-5-yl)oxy-6-methoxyquinolin-7-yl]oxy]methyl]cyclopropylaminedi Hydrochloride (referred to as the dihydrochloride of Compound I); Methotrexate; Cyclophosphamide; Oxaprozin.
[0071] Preparation method:
[0072] All the above samples were dissolved in dimethyl sulfoxide to prepare a 100 mmol / L mother solution, which was stored at -20°C for later use. When used, it was prepared with DMEM serum culture medium to the desired concentration. The dihydrochloride diluent of compound I was mixed with methotrexate, cyclophosphamide, and oxaprozin diluent respectively to determine whether the compound I dihydrochloride had better effect after combination.
[0073] Cell Culture:
[0074] The test cells were cultured in 10% fetal bovine serum and 0.1 g / L streptomycin and penicillin (final conc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com