Application of acetic anhydride serving as acyl donor to participation in DBAT (deacetyl baccatin acetyloxy transferase) enzymatic reaction
An acyl donor and enzymatic reaction technology, applied in the application field of acetic anhydride as a novel acyl donor participating in the DBAT enzymatic reaction, can solve the problem of high cost, no great economic benefit in industrialized production, and natural substrate acetyl coenzyme. A is expensive and other problems, to achieve great economic value and reduce the cost of industrial applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1. Method:
[0022] 1.1 Expression and purification of recombinant protein
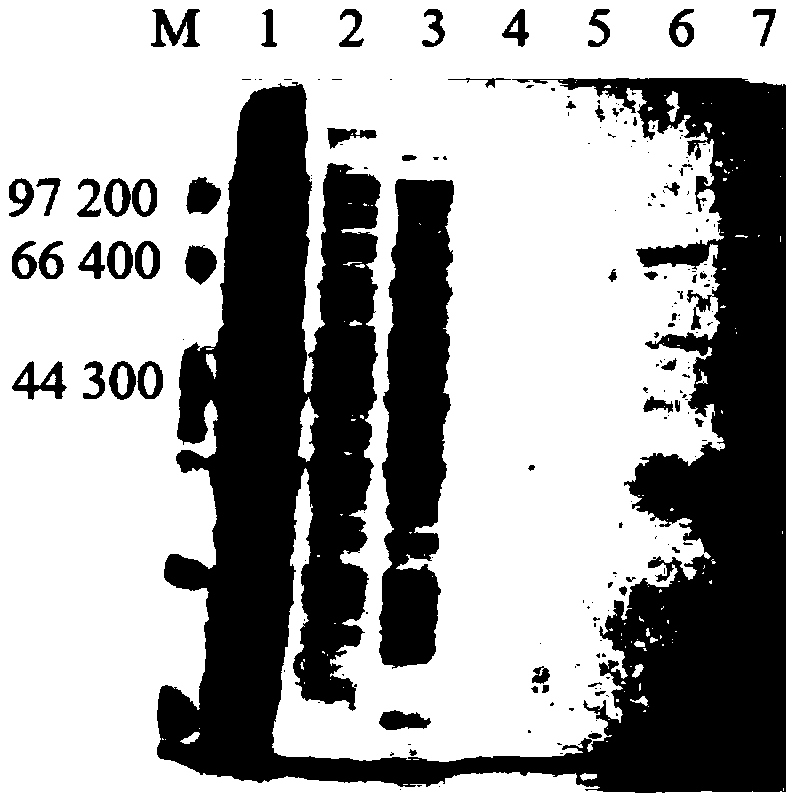
[0023] Pick a single colony of TBL21-DBAT transformed with the pET-DBAT plasmid and inoculate it into a 5 mL medium containing 100 μg·mL -1 Incubate overnight at 37°C in LB liquid medium with ampicillin. Pipette 1mL of bacterial liquid into 100mL of LB liquid medium, culture at 37°C and 220rpm for 2.5h to D 600nm After reaching 0.6-0.8, add IPTG with a final concentration of 0.1mmol / L, and induce culture at 20°C and 120rpm for 18h. Centrifuge at 8000rpm at 4°C for 15 minutes to collect the cells, add phosphate buffer (PBS, pH 7.4) to wash the cells twice, and finally add 10 mL of phosphate buffer to each 100 mL of the original fermentation liquid cells to resuspend, and vortex to shake Mix it thoroughly. Use an ultrasonic cell disruptor to crush the resuspended bacteria, set the ultrasonic power to 400W, and the total time is 12min, with an interval of 6s for every 6s. During the ultrasonic...
Embodiment 2
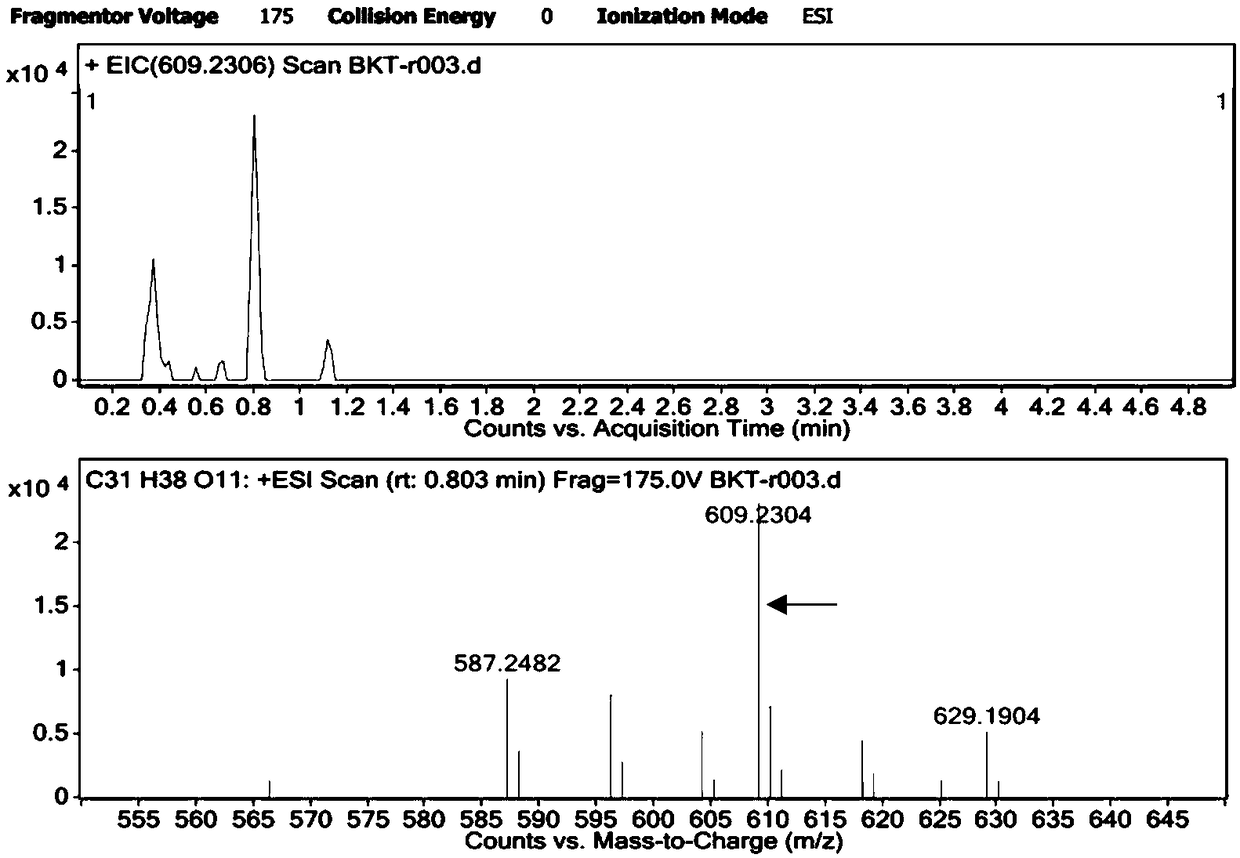
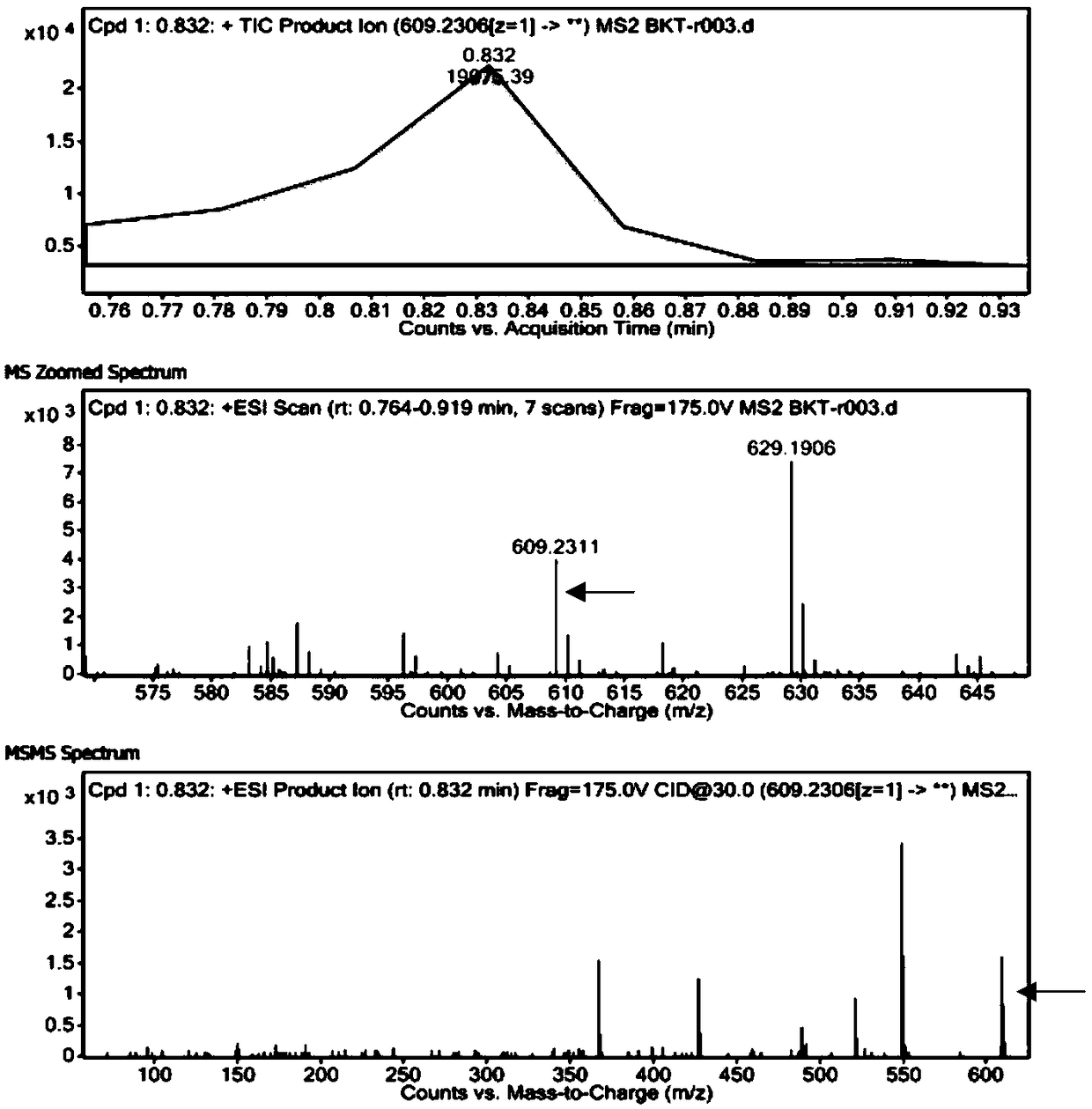
[0032] With reference to the reaction system in method 1.2 in Example 1, utilize high-performance liquid chromatography (HPLC) to detect the DBAT enzyme activity when respectively using acetyl CoA and acetic anhydride as substrates, and the liquid chromatography conditions are: C18 reverse column ( 5μm, 250mm×4.6mm), the detection wavelength is 227nm, the flow rate is 1mL / min, the column temperature is 30°C, the mobile phase is acetonitrile and water (40:60), and the injection volume is 20μL. A standard curve was prepared for the bacatine III standard under the same conditions, and the sample was quantitatively analyzed by the area external standard method.
[0033] Result analysis:
[0034] Comparison of enzyme activities under different substrates
[0035] The DBAT enzyme activity was measured separately when acetyl CoA and acetic anhydride were used as acyl donors to participate in the enzymatic reaction. The results are shown in Table 1. When acetic anhydride was used as ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com