Application of nometin in preparation of drug for ameliorating liver injury caused by cholestasis and metabolic diseases
A technology for metabolic diseases and liver damage, which can be used in drug combinations, pharmaceutical formulations, organic active ingredients, etc., and can solve the problems of complex pathogenesis of metabolic diseases.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] animal model
[0127] High-fat diet-induced obese mice (DIO) experiment.
[0128] Mouse Modeling and Grouping
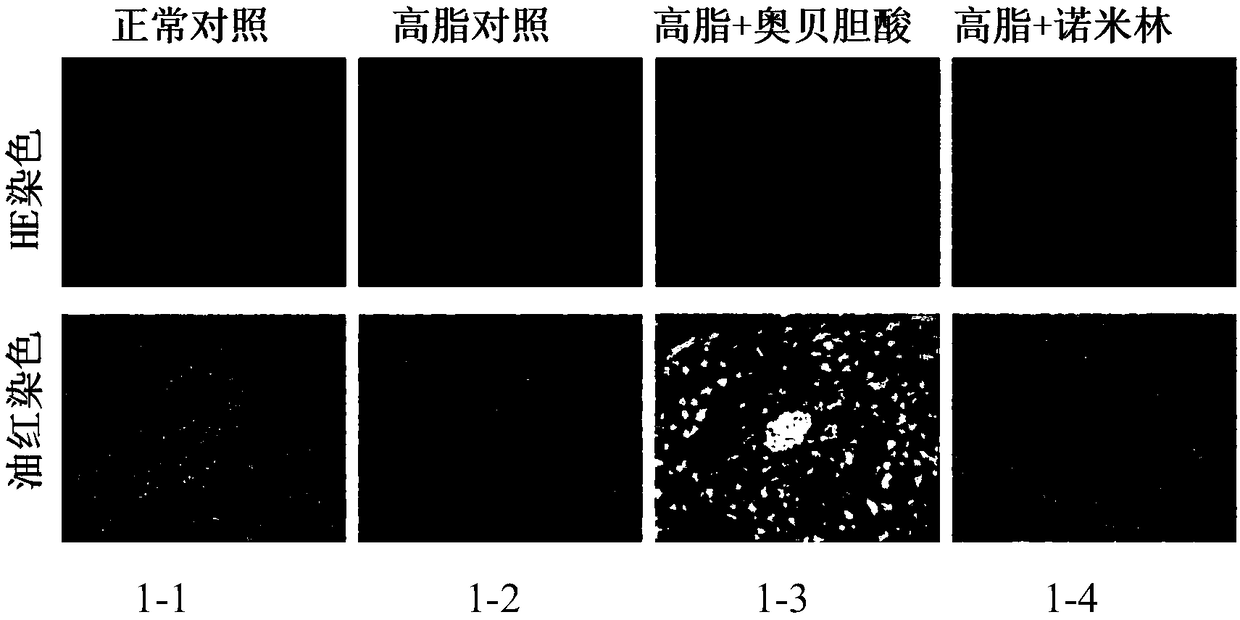
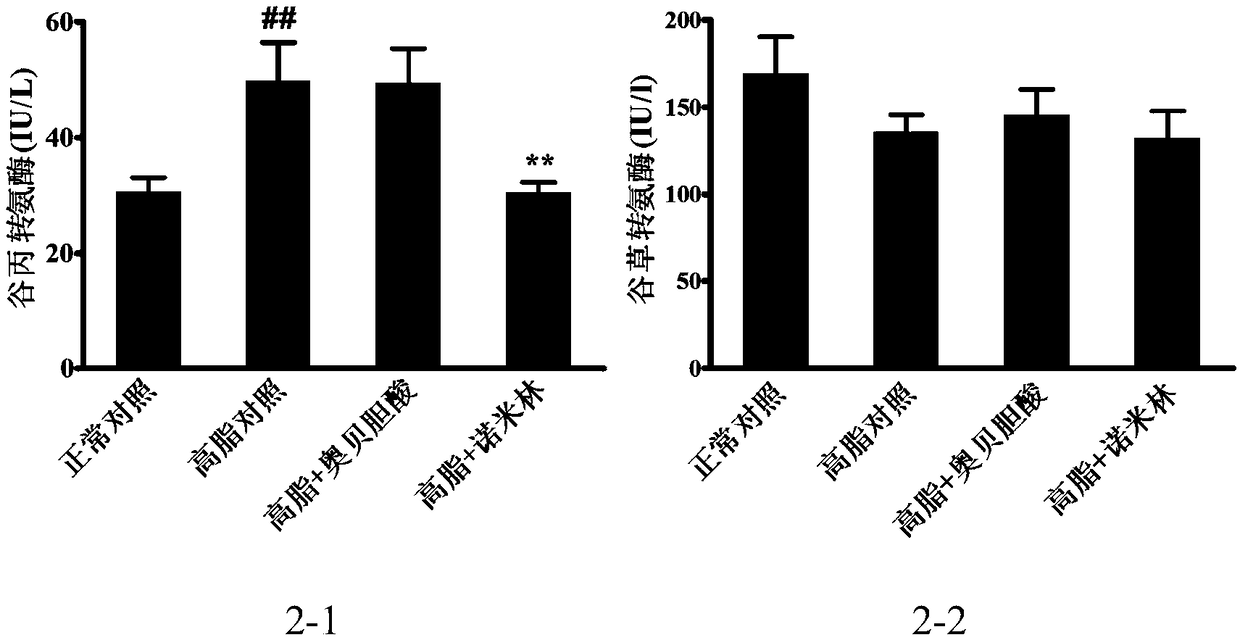
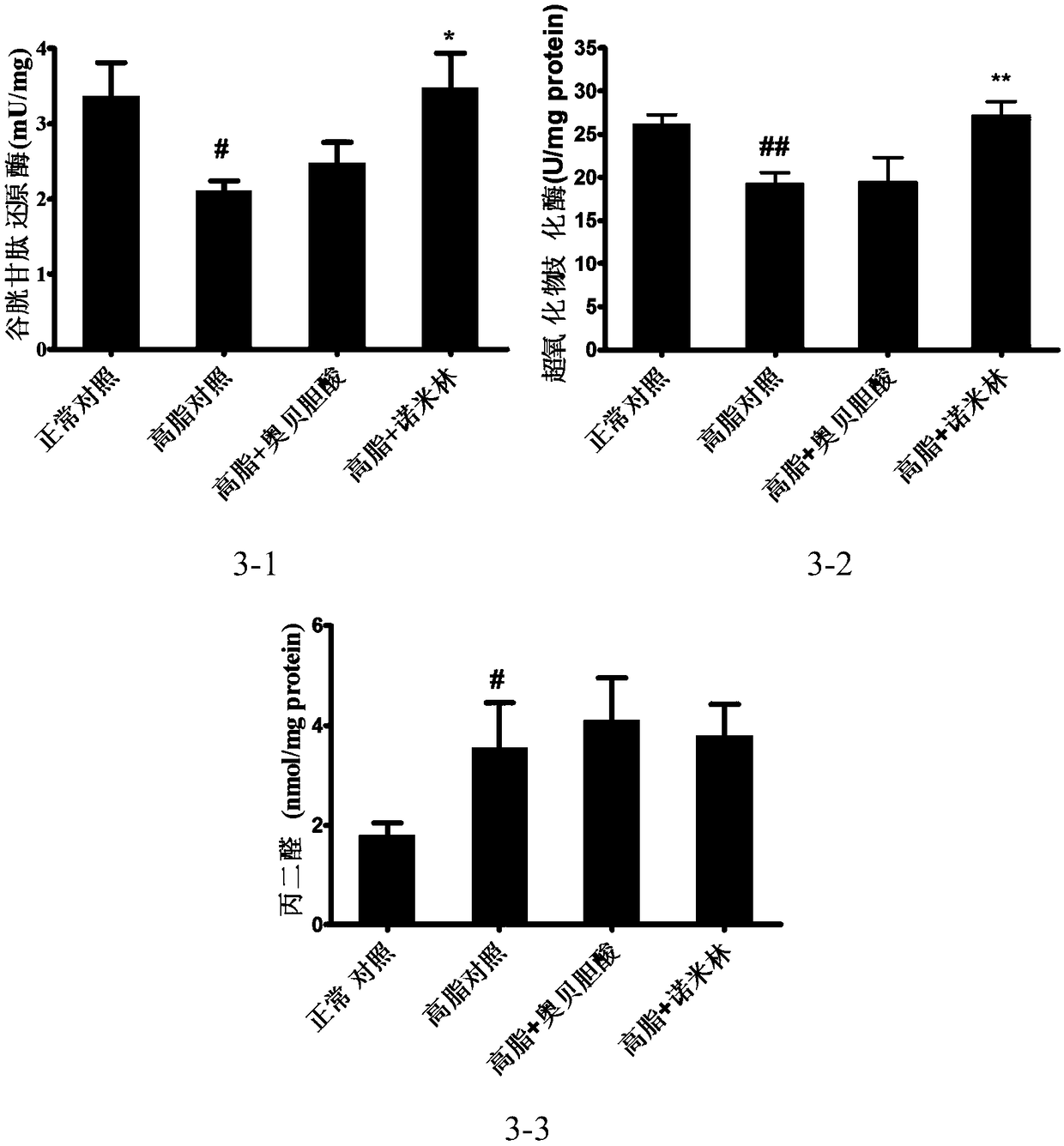
[0129] 7-week-old, C57BL / 6SPF female mice were acclimatized in the animal room for 1 week, and the Chow group was given a normal diet, and the rest were given a high-fat diet to induce obesity for 3 months. Finally, 30-40g obese mice that had been successfully modeled were selected, randomly divided into groups according to body weight, 8 mice in each group, and analyzed by SPSS21.0 software. There was no difference in body weight among the groups. Divided into: normal control group, high fat control group, high fat + obeticholic acid group, high fat + nomilin group.
[0130] Dosage and method of administration
[0131] Obeticholic acid: 15mg / kg Bodyweight / day (0.2 / 1000 high-fat feed)
[0132] Nomilin: 75mg / kg Bodyweight / day (1 / 1000 high-fat feed)
[0133] According to the above dosage, first mash the high-fat feed, then add the drug powder, mix well, and...
Embodiment 2
[0140] OB / OB Leptin Deficient Obese Mice Experiment
[0141] Mouse Modeling and Grouping
[0142] ob / ob mice, 12 weeks old, female, SPF level, weighing about 80g. All were given normal feed. According to the body weight, the rats were randomly divided into 6 groups, analyzed by SPSS21.0 software, and there was no difference in body weight among the groups. Divided into: normal group, ob / ob model group, ob / ob+nomilin (75mg / kg)
[0143] Dosage and method of administration
[0144] Gavage administration, once a day, continuously for one month, drug nomilin: 75 mg / kg, dissolved in 0.5% CMCNa, and ultrasonically prepared into a suspension in an ultrasonic instrument.
[0145] Effects of nomilin on the liver of ob / ob mice
[0146] Pathological analysis of liver HE staining in ob / ob mice: Compared with normal mice, the liver fatty degeneration of mice in the model group was very serious, and vacuolar deformation appeared (HE staining of ob / ob mouse liver and the content of liver...
Embodiment 3
[0148] MCD feed-induced steatohepatitis (NASH) mice experiment
[0149] Mouse Modeling and Grouping
[0150] 7-week-old, C57BL / 6SPF female mice were fed to 20-25g in the animal room of Shanghai University of Traditional Chinese Medicine. After the normal group was given MCS feed, the rest were all fed MCD feed. After 3 weeks of modeling, they were randomly divided into groups according to body weight. Eight rats in each group were analyzed by SPSS21.0 software, and there was no difference in body weight among the groups. Divided into: MCS control group, MCD model group, obeticholic acid group, nomilin (75mg / kg).
[0151] Dosage and method of administration
[0152] Gavage administration, once a day, continuous gavage for 4 weeks. Nomilin: 75mg / kg, dissolved in 0.5% CMCNa, and made into a suspension by sonication.
[0153] Nomilin ameliorates hepatic steatosis in mice induced by MCD diet
[0154] In order to further study the therapeutic effect of nomilin on alcoholic fatt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com