Application of Nomilin in Preparation of Drugs for Improving Liver Damage Caused by Cholestasis and Metabolic Diseases
A technology for metabolic diseases and cholestasis, which can be used in drug combinations, pharmaceutical formulations, organic active ingredients, etc., and can solve the problems of complex pathogenesis of metabolic diseases.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Animal model
[0127] High-fat feed-induced obesity mice (DIO) experiments.
[0128] Mouse modeling and grouping
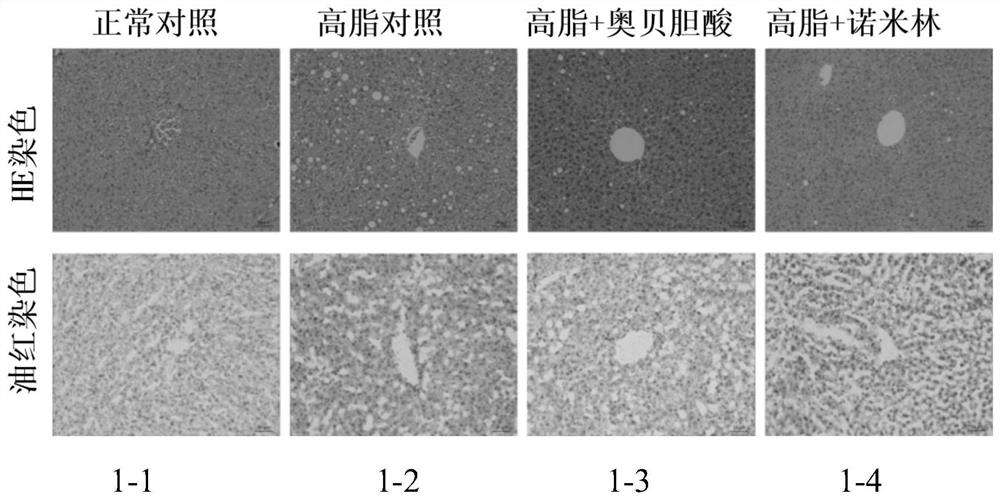
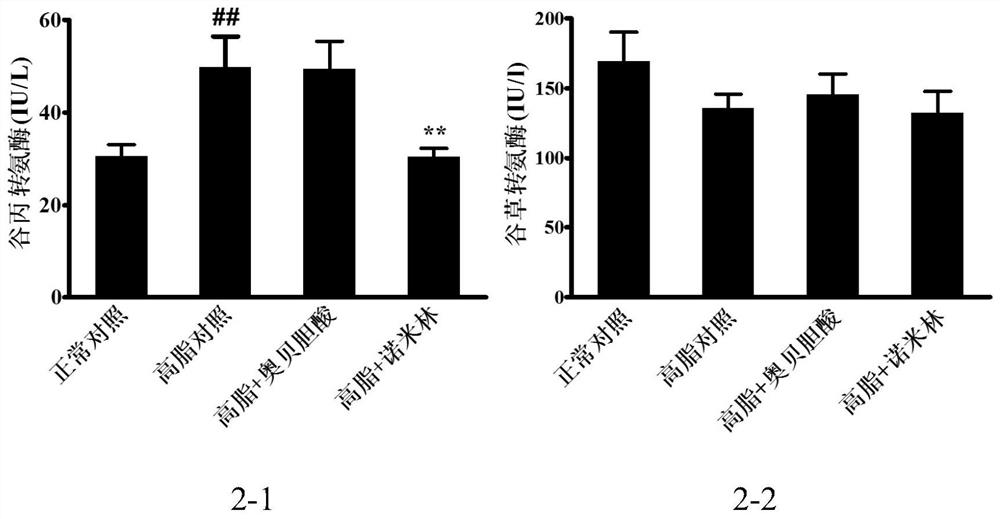
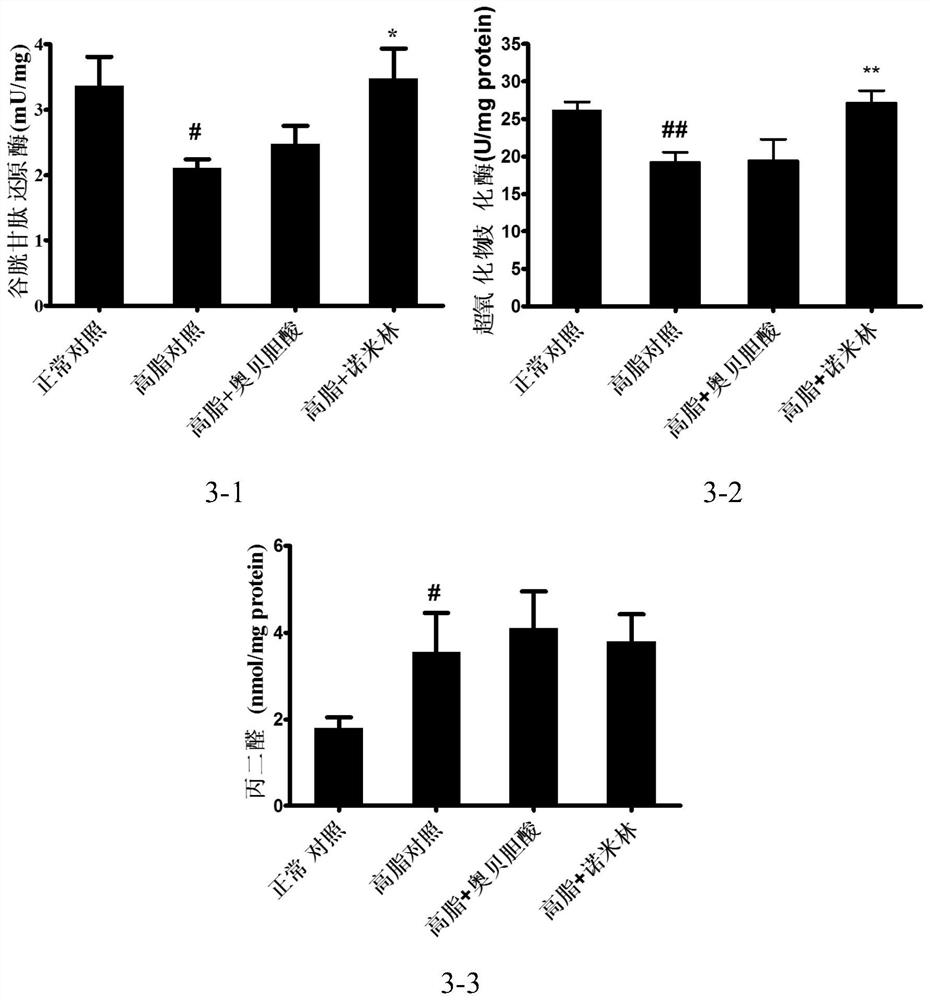
[0129] 7 weeks old, C57BL / 6SPF female mice, after 1 week of the animal room, leaving the CHOW group to give normal feed, and the rest of the high-fat feed induced obesity and induced for 3 months. After picking up 30-40 g of obesity mice that have been successfully molded, 8 of the groups, 8 SPSS21.0 software analysis, and there is no difference in weight between each group. Divided into: normal control group, high-fat control group, high fat + ovalcholic acid group, high fat + Nimin group.
[0130] Dosage dose and method
[0131] Obberry: 15mg / kg bodyweight / day (0.2 / 1000 high fat feed)
[0132] Nomin: 75mg / kg bodyweight / day (1 / 1000 high fat feed)
[0133] According to the above dose, first mash the high fat feed, then add the drug powder, mix evenly, make a cylindrical feed. Feeding the mice and administered for 7 weeks. During the period, the food...
Embodiment 2
[0140] Ob / OB leptin lack of obesity mouse experiment
[0141] Mouse modeling and grouping
[0142] Ob / OB mice, 12 weeks old, female, SPF level, with a weight of about 80g. All give ordinary feed. According to the weight random group, 6 of each group, SPSS21.0 software analysis, and there is no difference between the groups. Divided into: Normal group, OB / OB model group, OB / OB + Nimin (75mg / kg)
[0143] Dosage dose and method
[0144] Improving administration, once a day, continuously fed a month, drug Nomilin: 75 mg / kg, dissolved in 0.5% CMCNA, in the ultrasonic ultrasound to form a suspension.
[0145] Impact of Nimlin on the liver of OB / OB mice
[0146] By analyzing the pathological analysis of OB / OB mouse liver HE: Compared with normal mice, the hypoth of liver in the model group is very serious, and there is a void deformation (OB / OB mice HE staining and liver Tc, Tg content) Such as Figure 4 Disted, where Pic 4-1 The pathological sections of the liver hepati...
Embodiment 3
[0148] MCD feed-induced fatty hepatitis (NASH) mouse experiment
[0149] Mouse modeling and grouping
[0150] 7 weeks old, C57BL / 6SPF female mice, raised from 20-25g of animal houses in Shanghai Traditional Chinese Medicine to remove the normal group to give MCS feed, and all of the remaining MCD feeds, 3 weeks of modeling, according to the weight random group, Each group, SPSS21.0 software analysis, and there is no difference in weight between each group. Divided into: MCS control group, MCD model group, Obecholic acid group, Nimin (75mg / kg).
[0151] Dosage dose and method
[0152] Irrush, once a day, 4 weeks. Nomin: 75 mg / kg, dissolved in 0.5% CMCNA, and the ultrasonic ultrasound is used as a suspension.
[0153] Nomin improves the liver fat degeneration of MCD feed induced mice
[0154] In order to further study the therapeutic effect of Namilin's alcoholic fatty liver, we used MCD feed-induced fatty hepatitis model NASH, induced by C57BL / 6 after 3 weeks of MCD, admini...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com