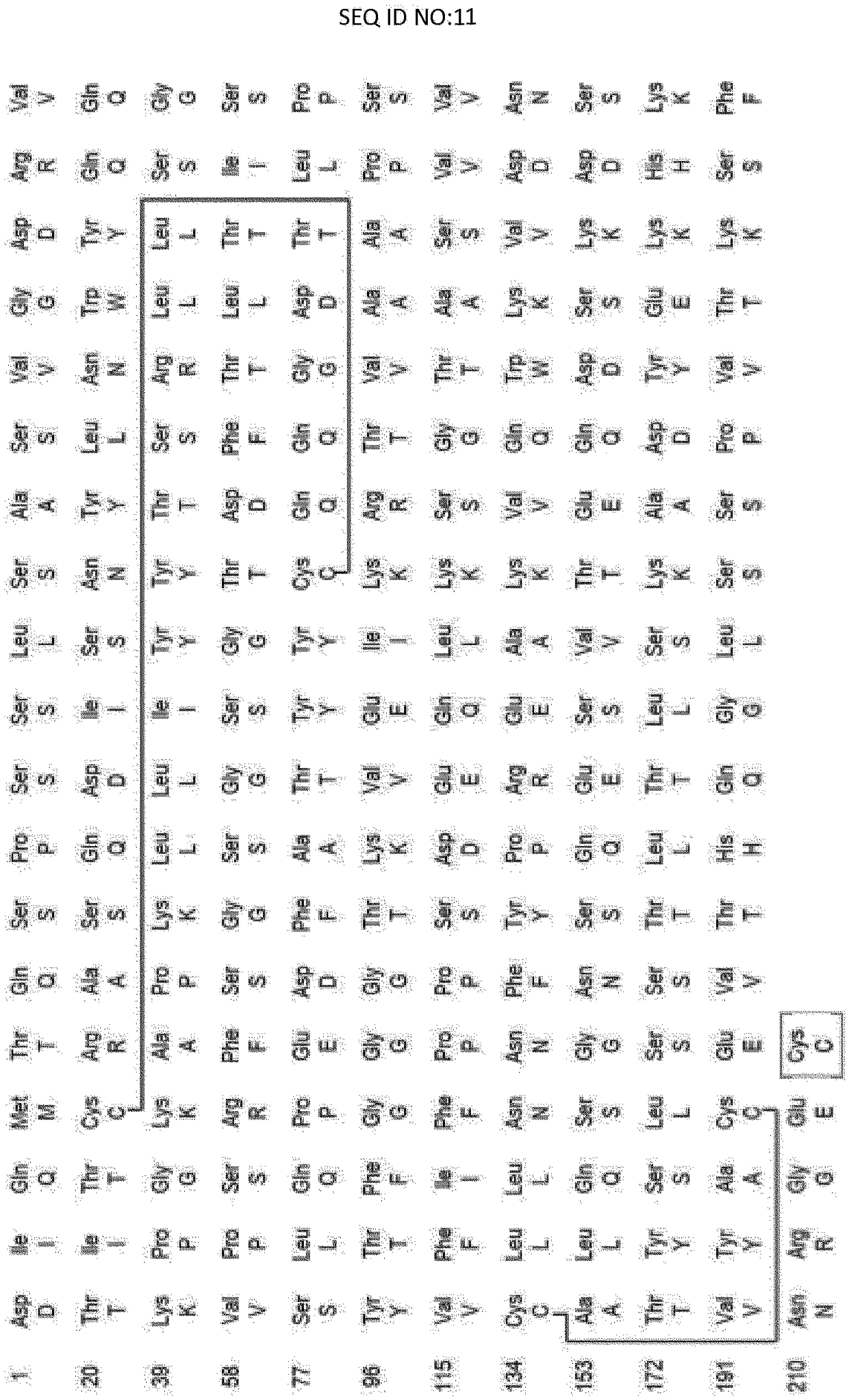
Pharmaceutical formulation
A preparation and liquid preparation technology, applied in the field of pharmaceutical preparations, can solve problems such as reduced antibody activity, blockage, and hindrance to the proper administration of pharmaceutical preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Example 1: Hydroxypropyl-γ-cyclodextrin (HPGCD) Screening
[0128] Stability and the propensity to precipitate in the formulation are greatly influenced by the concentration (steric crowding) and excipients used in the formulation, which control the interactions observed due to steric crowding.
[0129] To determine whether HPGCD has an effect on precipitation characteristics at physiological pH, a base formulation of romosumumab at a concentration of 120 mg / ml (pi 6.9) with and without HPGCD was run through the pH shift model.
[0130] A pH shift model was designed to replicate the pH shift that occurs when a drug-containing formulation is administered by subcutaneous injection (Elena García-Fruitós, Insoluble Proteins: Methods and Protocols, Methods in Molecular Biology, Vol. 1258, Chapter 18: 321-330, Springer Science+Business Media New York (2015)). Biotherapeutic proteins, especially antibodies, are typically formulated at physiological pH (~pH 7.0). If the pi ...
Embodiment 2
[0141] Example 2: Methionine Screening
[0142] Once the effect of HPGCD on the precipitation characteristics of the basic romoxomab formulation of Example 1 was assessed, the effect of methionine was investigated.
[0143] To determine whether methionine has an effect on precipitation characteristics at physiological pH, the pH shift model was run with (55 mM and 200 mM) and without methionine at a concentration of 90 mg / ml (control only ), 120 mg / mL or 180 mg / ml romoxomab base formulation (pI 6.9).
[0144] Romoxomab in 50 mM sodium acetate, 14 mM calcium acetate, 6% sucrose, 0.006% polysorbate 20 (pH 5.2) (drug solution, DS) was concentrated to 125 mg / ml using a 30 kDa Sartorius Viva Spin centrifugal spin filter. ml or to 190mg / ml. Spin tubes were centrifuged at 3000 rpm, 5°C for < 60 minutes; samples were mixed between spin cycles using positive displacement pipettes.
[0145] The required amount of methionine (0.018 g and 0.0066 g, respectively) to produce 2.2 mL of sa...
Embodiment 3
[0158] Example 3: Combination of HPGCD and Methionine Screening
[0159] Once the effect of HPGCD alone or methionine alone on the precipitation characteristics of the basic romoxomab formulation of Example 1 was assessed, the combined effect of HPGCD and methionine was investigated.
[0160] Romoxomab was concentrated to 125 mg in 50 mM sodium acetate, 14 mM calcium acetate, 6% sucrose, 0.006% polysorbate 20 (pH 5.2) (drug solution, DS) using a 30 kDa Sartorius Viva Spin centrifugal spin filter / ml or to 190mg / ml. Spin tubes were centrifuged at 3000 rpm, 5°C for < 60 minutes; samples were mixed between spin cycles using positive displacement pipettes.
[0161] The amount of methionine (0.018 g and 0.066 g, respectively) required to produce 2.2 mL of sample with the desired concentration (55 and 200 mM) was weighed using an analytical balance. Dissolve methionine in 2.2 mL of the appropriate protein solution (125 mg / ml or 190 mg / ml). The required amount (0.053 g) of HPGCD...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com