Smc combination therapy for treatment of cancer
A cancer, VSV-M51R technology, applied in the field of treatment of patients diagnosed with cancer, can solve problems such as insufficient treatment of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0224] Example 1: Smac Mimetics Trigger Tumor Destruction by the Innate Immune System
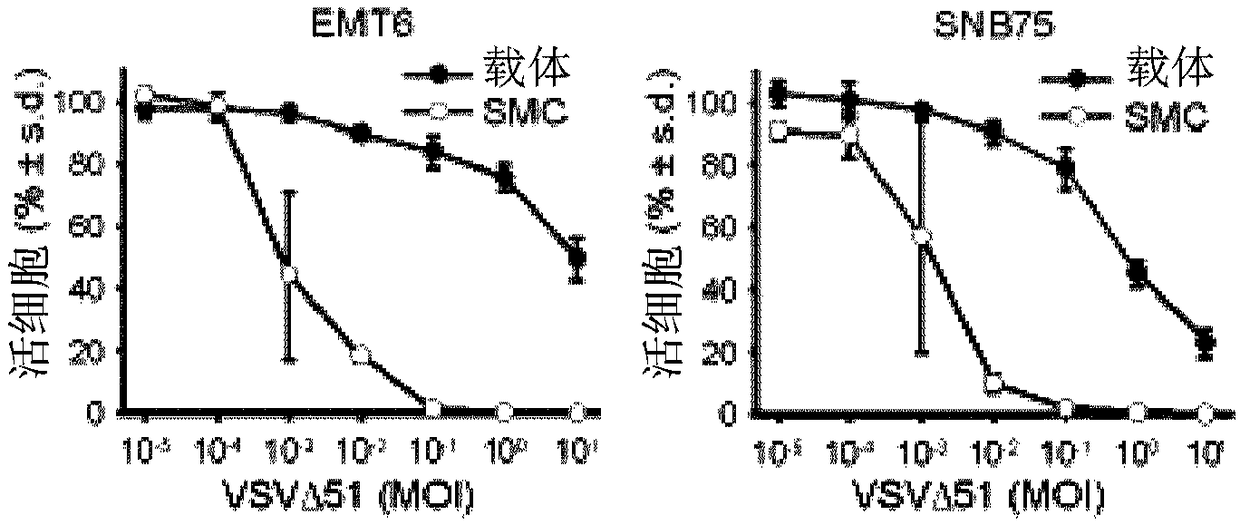
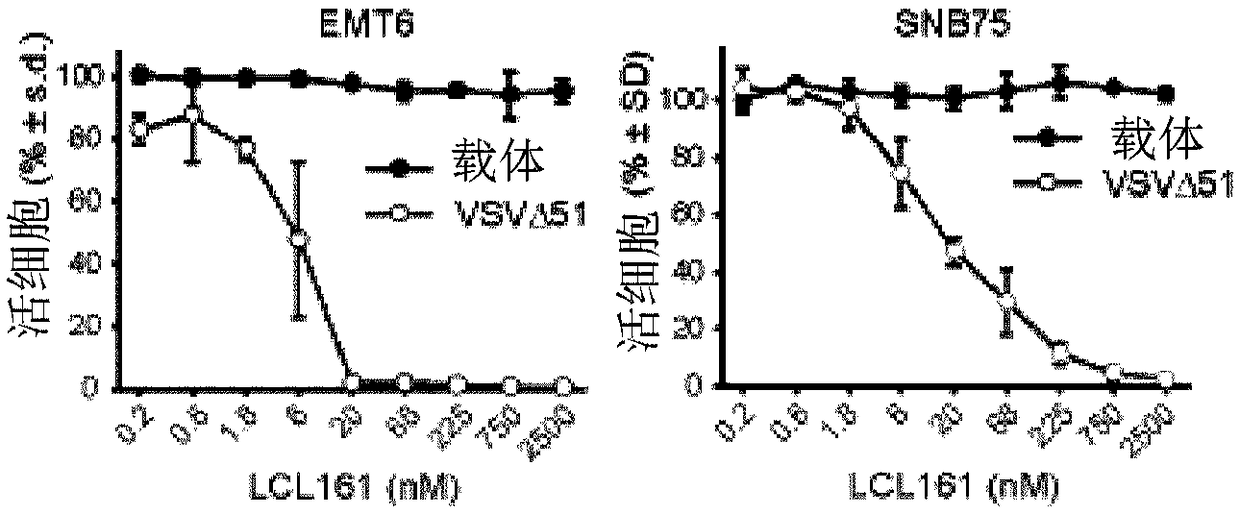
[0225] Smac mimetic compounds, a class of apoptosis-sensitizing drugs, have been shown to be safe in phase I trials in cancer patients. Stimulation of the innate antipathogen response can generate an effective and safe inflammatory "cytokine storm" that triggers the death of tumors treated with Smac mimics. This example demonstrates the activation of innate immune responses by oncolytic viruses and adjuvants such as poly(I:C) and CpG to induce bystanders in cancer cells treated with Smac mimetics in a manner mediated by IFNβ, TNFα or TRAIL cell death. This therapeutic strategy can lead to durable cures, for example, in several mouse models of aggressive cancers. As these and other innate immune stimulants have been shown to be safe in human clinical trials, the data presented here strongly point to their use in combination with Smac mimetics for the treatment of cancer.
[0226] This exa...
Embodiment 2
[0254] Example 2: Inactivated Virus Particles, Cancer Vaccines and Stimulatory Cytokines Synergize with SMCs to Kill Tumors
[0255] Current cancer immunotherapies such as BCG (Bacillus Calmette-Guerin), recombinant interferons (eg IFNα) and recombinant tumor necrosis factor (eg TNFα for isolated limb perfusion), and more recently clinically used biologics ( The application of immune checkpoint inhibitors (such as anti-CTLA-4 and anti-PD-1 or PDL-1 monoclonal antibodies) to overcome tumor-mediated suppression of the immune system highlights "cancer immunotherapy" as an effective potential for therapeutic forms. As shown in Example 1, we have demonstrated the strong potential of non-viral immunostimulants to act synergistically with SMCs (Figure 5). To extend these studies, we also examined the potential of SMCs to synergize with non-replicating rhabdovirus-derived particles (termed NRRPs), which are UV-irradiated particles that retain their infectivity and immunostimulatory p...
Embodiment 3
[0305] Example 3: Immunotherapy containing SMC shows anti-myeloma activity
[0306] Immune checkpoint blockade synergizes with SMC therapy to delay disease progression in MM
[0307] MPC-11 cells stably expressing the luciferase transgene were implanted into BALB / c mice by intravenous injection. This in vivo MM model mimics human disease well and follows predictable disease progression. MPC-11 cells were obtained from murine plasmacytoma. After two rounds of treatment with SMCs and monoclonal antibodies against PD-1 or CTLA-4, only anti-PD-1-based therapy showed a response with delayed disease progression. Mice treated with the combination of anti-PD-1 and SMC showed the best response with little tumor burden as determined by luminescent signal (Figure 35). The combination also significantly prolonged the survival of mice compared to the control group (p=0.01), and compared to PD-1 treatment alone (p=0.0163).
[0308] Type 1 interferons cooperate with SMCs to cause MM cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com