Preparation method of high-conductivity metal net load graphene current collector
A high-conductivity, metal mesh technology, used in electrode carriers/current collectors, circuits, electrical components, etc., can solve problems such as large current collector resistance, and achieve the effect of improving anti-oxidation performance and high conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] 1) Raw material pretreatment: the commercial metal used is copper; the graphene used is graphene, and the oxide layer on the surface of the metal mesh is cleaned with dilute acid, which is dilute hydrochloric acid.
[0054] 2) Ultrasonic dispersion treatment of graphene: ultrasonically disperse a certain amount of graphene in deionized water for 20 minutes.
[0055] 3) Reaction temperature: hydrothermal reaction temperature 120°C, reaction time 24h.
[0056] 4) Cleaning and drying conditions: Rinse with deionized water until there are no tiny graphene particles adsorbed on the surface. Dry at 60°C for 6h under vacuum.
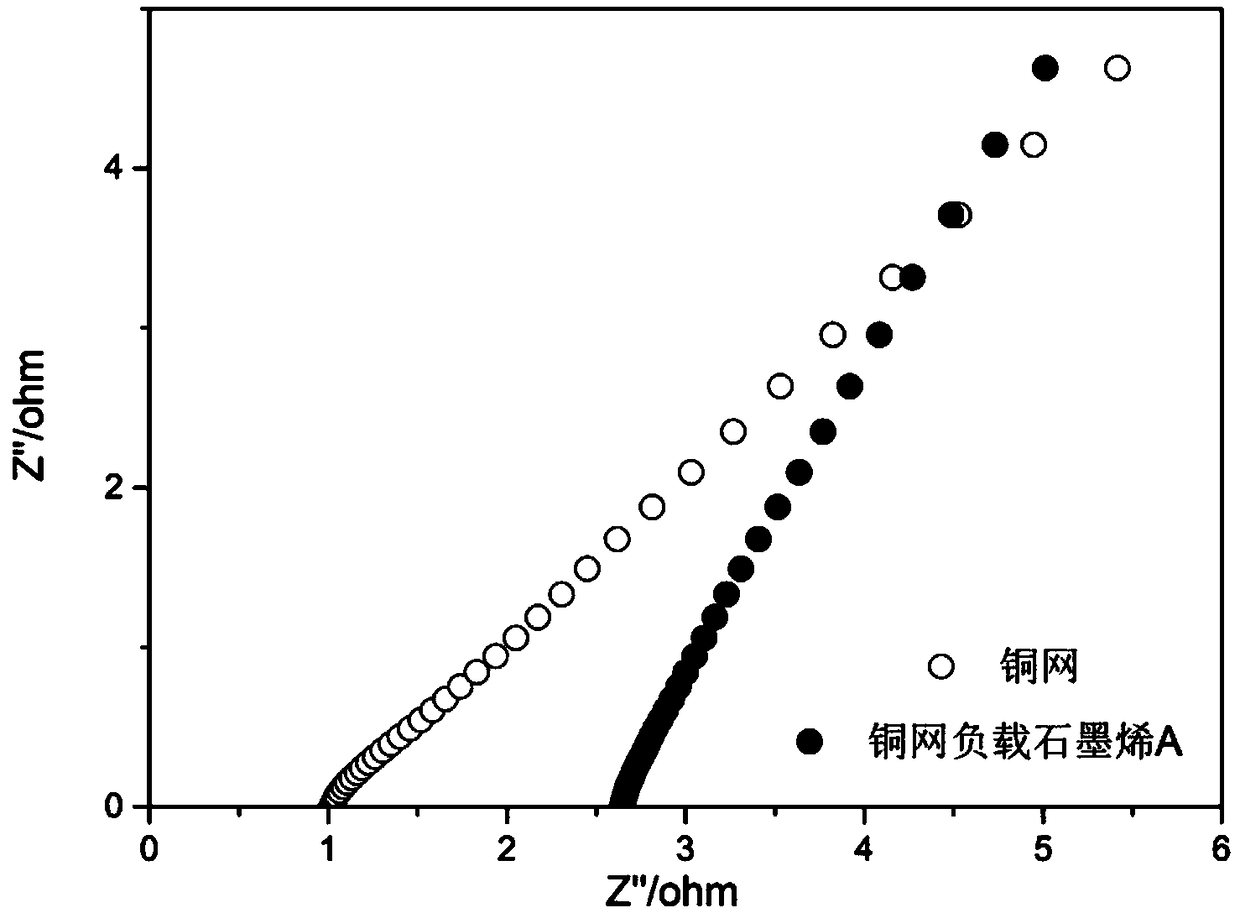
[0057] After testing with a multimeter, the resistance of the copper current collector was 0.2Ω before recombination, but after recombination, its resistance was significantly lower than 0.1Ω, exceeding the lower limit of the measurement range of the multimeter. The electrochemical impedance spectroscopy (Electrochemical Impedance Spectroscopy, EIS) of...
Embodiment 2
[0059] 1) Raw material pretreatment: the commercial metal used is copper; the graphene material used is graphene oxide; the oxide layer on the surface of the metal mesh is cleaned with dilute acid, which is an acetic acid solution.
[0060] 2) Ultrasonic dispersion treatment of graphene: ultrasonically disperse a certain amount of graphene in deionized water for 60 minutes.
[0061] 3) Reaction temperature: the hydrothermal reaction temperature is 200° C., and the reaction time is 12 hours.
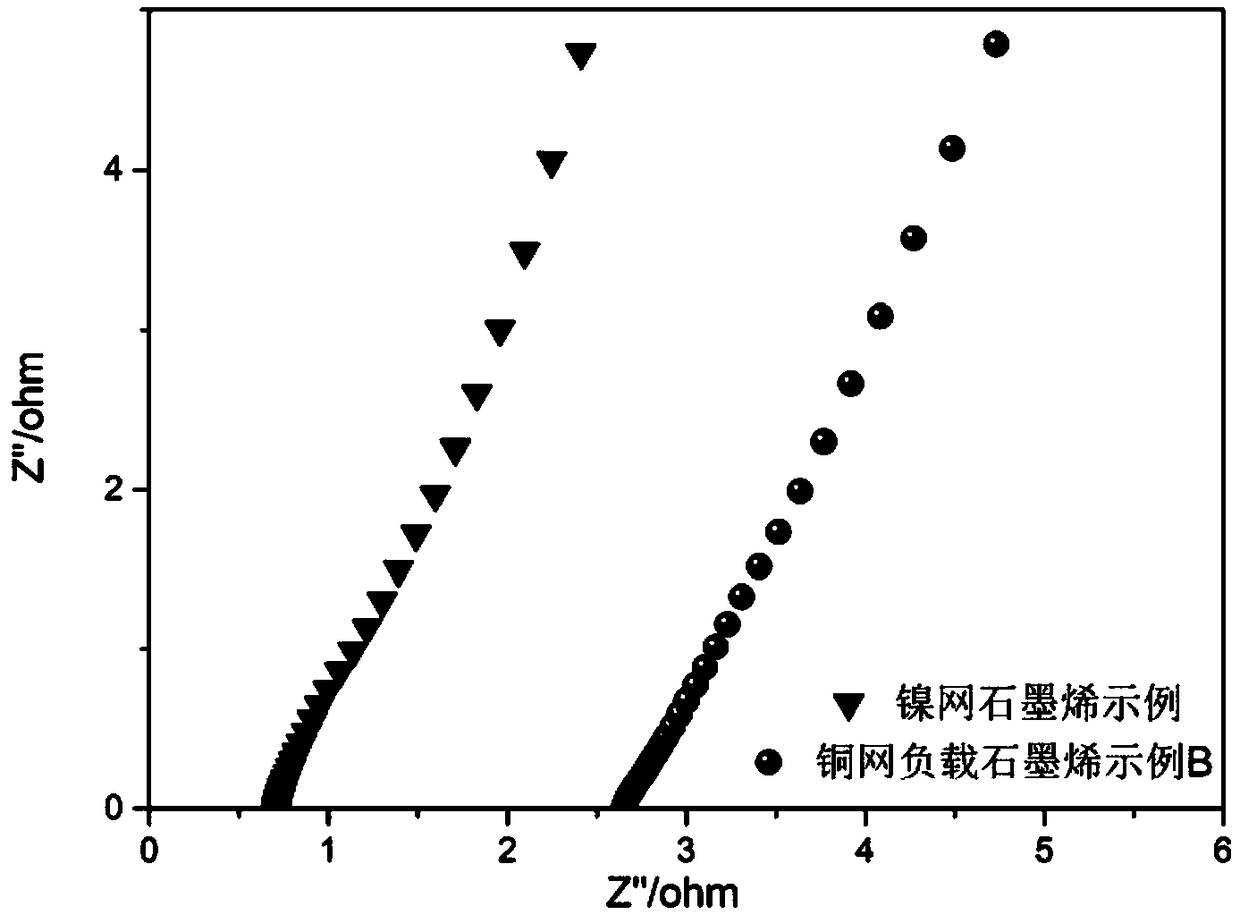
[0062] 4) Cleaning and drying conditions: Rinse with deionized water until there are no tiny graphene particles adsorbed on the surface. Dry in air for 24h. Its electrochemical impedance spectroscopy as image 3 As shown, it can be seen that this example B ratio of copper mesh loaded graphene figure 2 The slope of graphene-supported copper grid example A is slightly increased, indicating that this scheme has lower resistance characteristics in electrochemical systems.
Embodiment 3
[0064] 1) Raw material pretreatment: the commercial metal used is still metal nickel; the graphene material used is reduced graphene oxide; the oxide layer on the surface of the metal mesh is cleaned with dilute acid, which is dilute hydrochloric acid.
[0065] 2) Ultrasonic dispersion treatment of graphene: ultrasonically disperse a certain amount of graphene in deionized water for 30 minutes.
[0066] 3) Reaction temperature: the hydrothermal reaction temperature is 140° C., and the reaction time is 18 hours.
[0067] 4) Cleaning and drying conditions: Rinse with deionized water until there are no tiny graphene particles adsorbed on the surface. Dry in air for 24h. Electrochemical impedance spectroscopy of nickel mesh loaded with graphene such as image 3 As shown, it can be seen that the slope of nickel mesh loaded with graphene is roughly the same as that of copper mesh loaded with graphene, which indicates that graphene is evenly loaded on the surface of the two metals ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com