Preparation method of lithium difluorophosphate
A technology of lithium difluorophosphate and lithium hexafluorophosphate, applied in chemical instruments and methods, lithium halides, phosphorus compounds, etc., can solve the problems of heavy metal impurity sources, limitations, and high requirements for reaction conditions, and achieve stable and easy-to-control reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0023] The preparation method of the lithium difluorophosphate of the embodiment of the present invention comprises the following steps:
[0024] 1) dissolving lithium hexafluorophosphate in an organic solvent to obtain a first solution, and adjusting the temperature of the first solution to 20-30°C;
[0025] 2) Dissolving lithium hydroxide in an organic solvent to obtain a second solution, adjusting the temperature of the second solution to 15-30°C;
[0026] 3) adding the second solution dropwise to the first solution to react to obtain a reactant;
[0027] 4) performing solid-liquid separation on the reactant to obtain a lithium difluorophosphate solution;
[0028] 5) Filtering the lithium difluorophosphate solution, crystallizing to obtain lithium difluorophosphate crystals, and drying to obtain a finished product.
[0029] In order to better understand the above technical solution, the technical solution of the present application will be described in detail below throug...
Embodiment 1
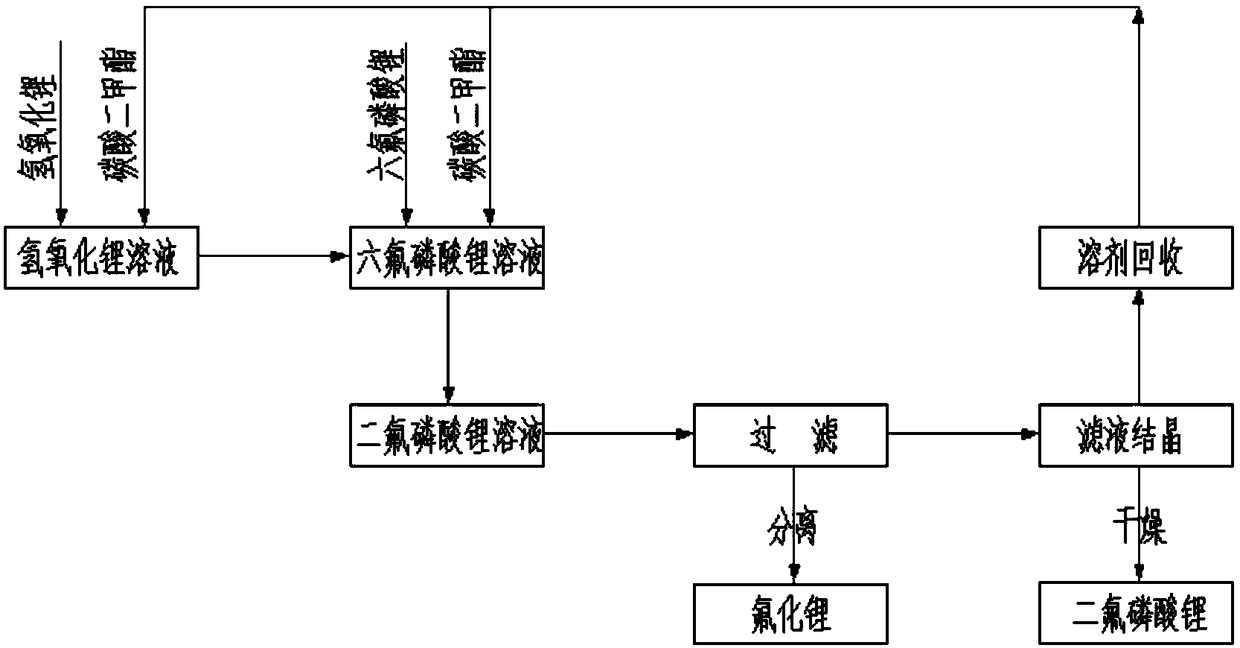
[0043] figure 1It is a process flow chart of the preparation method of lithium difluorophosphate in the embodiment of the present invention; the preparation method of lithium difluorophosphate in the present embodiment comprises the following steps:
[0044] Prepare lithium hexafluorophosphate solution: Take 40Kg of dimethyl carbonate liquid and add it to the reactor, slowly add 10Kg of lithium hexafluorophosphate, stir for 30 minutes after completion, and adjust the solution temperature to 20°C.
[0045] Prepare lithium hydroxide solution: Take 18Kg of dimethyl carbonate liquid and add it to the reactor, slowly add 2.66Kg of lithium hydroxide, stir for 30min after completion, and adjust the solution temperature to 15°C.
[0046] Add the lithium hydroxide solution dropwise to the lithium hexafluorophosphate solution at a rate of 10L / min, stirring continuously during this period. The dropping process takes about 3-4 hours in total. After the dropping is completed, continue to s...
Embodiment 2
[0053] The preparation method of lithium difluorophosphate of the present embodiment comprises the following steps:
[0054] Prepare lithium hexafluorophosphate solution: Take 800Kg of diethyl carbonate and add it to the reactor, slowly add 200Kg of lithium hexafluorophosphate, stir for 30 minutes after completion, and adjust the solution temperature to 20°C.
[0055] Prepare lithium hydroxide solution: Take 360Kg of diethyl carbonate and add it into the reactor, slowly add 53.2Kg of lithium hydroxide, stir for 30 minutes after completion, and adjust the solution temperature to 15°C.
[0056] Add the lithium hydroxide solution dropwise into the lithium hexafluorophosphate solution at a rate of 200 L / min, while stirring continuously. It takes about 3-4 hours in total. After the dropwise addition was completed, the lithium hexafluorophosphate solution was continuously stirred for 2 hours.
[0057] After the reaction is completed, the reactant is centrifuged to obtain a lithium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com