Preparation method of capecitabine molecularly imprinted sustained and controlled release material doped with poss and mcm-41-mps
A technology of MCM-41-MPS and capecitabine, which can be used in medical preparations with non-active ingredients, medical preparations containing active ingredients, organic active ingredients, etc., and can solve problems such as molecular recognition characteristics with limited effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
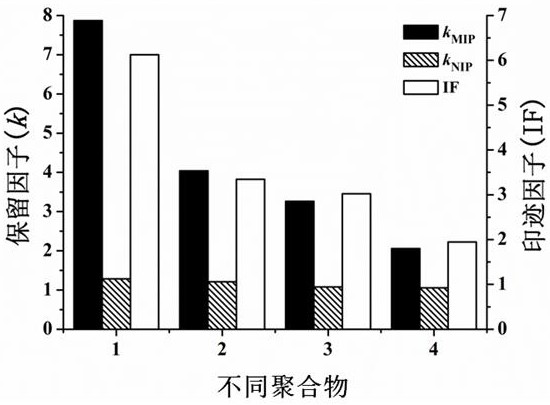
[0034] In order to confirm the effect of POSS and MCM-41-MPS on the imprinting effect of monolithic columns, we prepared different molecularly imprinted monolithic columns, one was MIP doped with POSS and MCM-41-MPS, and the control group was doped with POSS MIP, MIP doped with MCM-41-MPS and regular MIP. Through high-performance liquid phase analysis results, the specific operation steps are as follows:
[0035] Preparation steps:
[0036] First, the template molecule CAP (1.80%) and the initiator AIBN (0.57%) were dissolved in the binary porogen DMSO (31.06%) and [BMIM]BF 4 (32.87%), and then POSS (0.26%) was dissolved in the crosslinker EDMA (27.18%). The two mixtures were mixed and sonicated (5 min), then the binary monomers AAm (0.97%) and AMPS (2.36%) were added and dissolved by sonication. Finally, add MCM-41-MPS (0.26%) and sonicate for 5 minutes to disperse evenly. The pre-polymerization solution was introduced into a stainless steel column (100 × 4.6 mm inner dia...
Embodiment 2
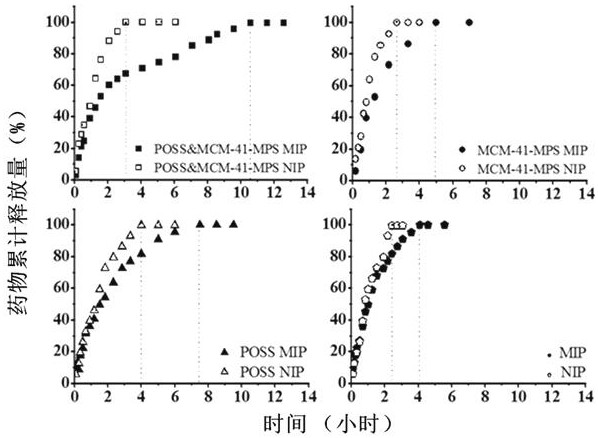
[0041] The effect of hybrid materials on the drug release effect of polymers was studied by in vitro drug release experiments. The results are shown in figure 2 and Table 1.
[0042] Table 1 In vitro drug release data
[0043]
[0044] The results showed that compared with the four groups of polymers, the release time of conventional MIP was the shortest, and the release amount of MIP reached the maximum value of 50 μg at about 4 h. However, the drug release amount of the polymer doped with POSS and MCM-41-MPS was significantly increased to 226 μg, and the drug burst rate was the lowest (3.1%), and the drug release time was also significantly extended to 11 h. This fully demonstrates that POSS and MCM-41-MPS MIP can significantly delay the release time of capecitabine, and have a good slow and controlled release effect on the drug.
Embodiment 3
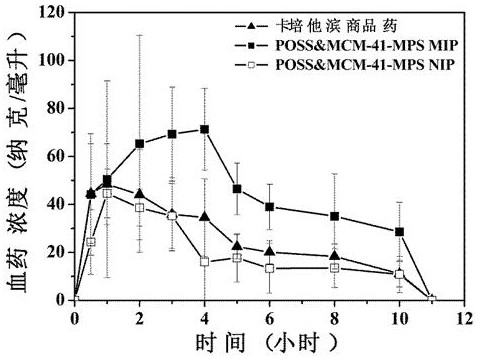
[0046] The drug release effect of the optimal formula POSS&MCM-41-MPS MIP and the control group (ie POSS&MCM-41-MPS NIP and CAP commercial drugs) were compared through pharmacokinetic experiments in rats. The results are shown in image 3 .
[0047] The results showed that the optimal formulation POSS&MCM-41-MPS MIP had the longest peak time (4h) and the largest peak area (483.7 ng h / mL), while the conventional MIP had the smallest peak time and peak area. Comparing the relative bioavailability, the relative bioavailability of different drug-loaded materials is quite different. The relative bioavailability of the best formulation was the highest, 173.4%, while the relative bioavailability of conventional MIP was the lowest, 72.5%. The relative bioavailability of AMPS MIP and AAm MIP in the control group were 99.4% and 83.0%, respectively. These results fully proved that the use of AAm and AMPS as binary monomers does have an impact on the drug release effect of the drug-loade...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com