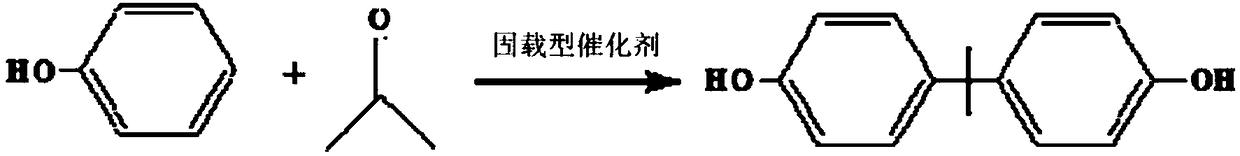
Supported catalyst for catalytic synthesis of bisphenol A and preparation method thereof
A catalyst and bisphenol technology, which is applied in the field of immobilized catalyst for catalyzing the synthesis of bisphenol A and its preparation, can solve unsatisfactory problems and achieve the effects of reducing equipment requirements, mild reaction conditions, and increasing dehydration condensation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] In addition, for the immobilized catalyst of the present invention, the applicant specifically provides a preparation method, comprising the following steps:
[0026] S101: Add the core-shell structured mesoporous nanospheres of the immobilized catalyst into the Erlenmeyer flask, add concentrated sulfuric acid with a preset ratio drop by drop while stirring, and then let stand for adsorption for 12 to 15 hours. Wherein, the mass ratio of the carrier to the concentrated sulfuric acid is 1:(6-8), and the mass percent concentration of the concentrated sulfuric acid is 80-85%.
[0027] S102: Centrifuge the solution obtained in S101, take the bottom precipitate, and dry at 100-130° C. for 4-8 hours.
[0028] S103: cooling the dried product, washing it in boiling absolute ethanol, and drying it in vacuum at 110-125° C. to obtain a solid-supported catalyst.
[0029] In addition, the solid-supported catalyst provided by the invention is used in the synthesis process of bisphen...
Embodiment 1
[0036] This example provides a solid-supported catalyst, which is used to catalyze the synthesis of bisphenol-A. specifically:
[0037] (1) Preparation of solid-supported catalyst
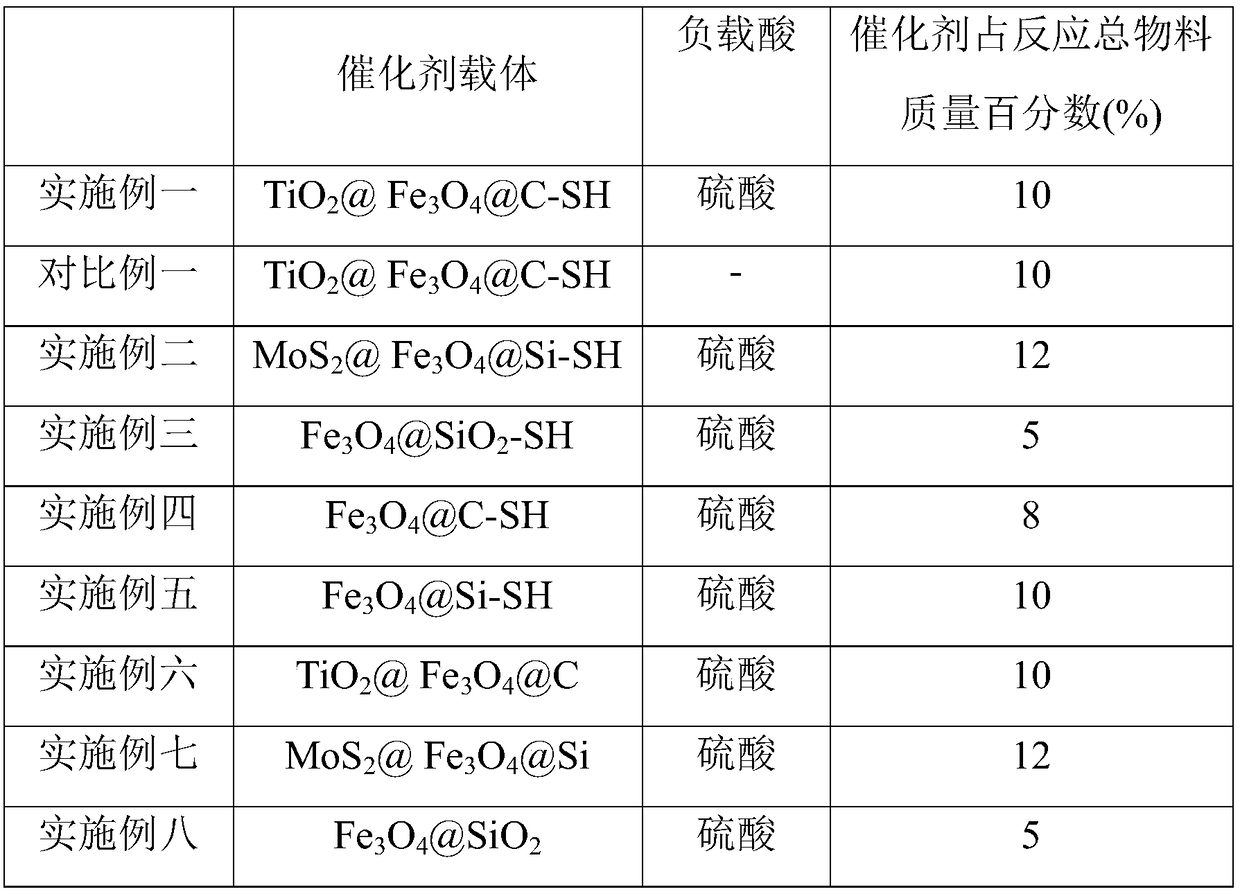
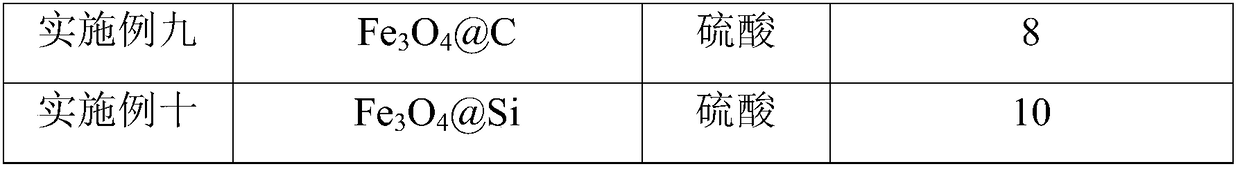
[0038] S101: Carrier-core-shell structure mesoporous nanosphere TiO 2 @Fe 3 o 4 Add @C-SH into the Erlenmeyer flask, add concentrated sulfuric acid with a mass percent concentration of 82% drop by drop while stirring, and then let it stand for adsorption for 15 hours. Wherein, the mass ratio of carrier to concentrated sulfuric acid is 1:7.
[0039] S102: Centrifuge the solution obtained from the treatment of S101, take the bottom precipitate, and dry it at 120° C. for 5 hours.
[0040] S103: cooling the dried product, washing it in boiling absolute ethanol, and drying it in vacuum at 120° C. to obtain a solid-supported catalyst.
[0041] (2) Synthesis of bisphenol A
[0042] S201: Mix phenol and acetone uniformly at a preset molar ratio of 1:5 (0.1mol:0.02mol) to obtain a reaction mixture; t...
Embodiment 2
[0047] This example provides a solid-supported catalyst, which is used to catalyze the synthesis of bisphenol-A. specifically:
[0048] (1) Preparation of solid-supported catalyst
[0049] S101: Carrier-core-shell structured mesoporous nanosphere MoS 2 @Fe 3 o 4 Add @Si-SH into the Erlenmeyer flask, add concentrated sulfuric acid with a mass percent concentration of 82% drop by drop while stirring, and then let it stand for adsorption for 12 hours. Wherein, the mass ratio of carrier to concentrated sulfuric acid is 1:6.
[0050] S102: Centrifuge the solution obtained from the treatment of S101, take the bottom precipitate, and dry it at 130° C. for 4 hours.
[0051] S103: cooling the dried product, washing it in boiling absolute ethanol, and drying it in vacuum at 110° C. to obtain a solid-supported catalyst.
[0052] (2) Synthesis of bisphenol A
[0053] S201: Mix phenol and acetone uniformly at a preset molar ratio of 1:1 (0.1mol:0.1mol) to obtain a reaction mixture; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com