High-molecular polymer containing perfluoro alkyl sulfimine side-chain and its synthesizing method
A technology of perfluoroalkylsulfonimide and high-molecular polymer is applied in the field of high-molecular polymer containing perfluoroalkylsulfonimide side chain and its synthesis, which can solve the problem of limited use range and poor heat resistance. and other problems, to achieve the effect of good catalytic effect, enhanced thermal stability, and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Synthesis of peralkylfluorosulfonimide side chain polyphosphazenes
[0027] (1). Chlorosulfonation of polyphenoloxyphosphazene
[0028] Weigh 1.24g of polyphenoloxyphosphazene and add 6ml of chloroform. Stir to dissolve it, then add 2.48g of chlorosulfonic acid under ice bath conditions, react at 60°C for 4h, add 5.5ml of thionyl chloride, continue the reaction for 1h, pour the mixture into a large amount of ice water, and precipitate a solid. The solid was washed with a large amount of distilled water until it became neutral, and then dried under reduced pressure to obtain 1.87 g of a white solid, that is, polyphenoloxyphosphazene after chlorosulfonation.
[0029] (2). Reaction of polybenzenesulfonyl chloride phosphazene with perfluorosulfonamide
[0030] Weigh 1.30 g of polyphenoloxyphosphazene after chlorosulfonation, add 5 ml of chloroform and 1.56 g of perfluoromethanesulfonamide, then slowly add 1.3 g of triethylamine dropwise, and react at 50° C. for...
Embodiment 2
[0036] Example 2: Synthesis of peralkylfluorosulfonimide side chain cross-linked polystyrene
[0037] (1) Weigh 20 g of cross-linked polystyrene, add 40 ml of 1,2-dichloroethane, add 60 g of chlorosulfonic acid, and react at 80° C. for 6 hours. After the reaction, the mixture was poured into ice water, the solid was washed to neutrality, dried in vacuum, and the obtained solid was washed with SOCl 2 After treatment, 33.6 g of chlorosulfonated polystyrene was obtained.
[0038] (2) Get the above-mentioned 4.90g chlorosulfonated polystyrene, 6.86g perfluorosulfonamide (C 4 f 9 SO 2 NH 2 ), in 25mlCH 2 ClCH 2 In the Cl solvent, add triethylamine (7g) dropwise, control the temperature at 80°C, and react for 48 hours. After the reaction, wash with 50ml dilute HCl, 50ml chloroform, and 250ml deionized water in sequence, extract and purify, and then dry under reduced pressure . The product was 9.68g.
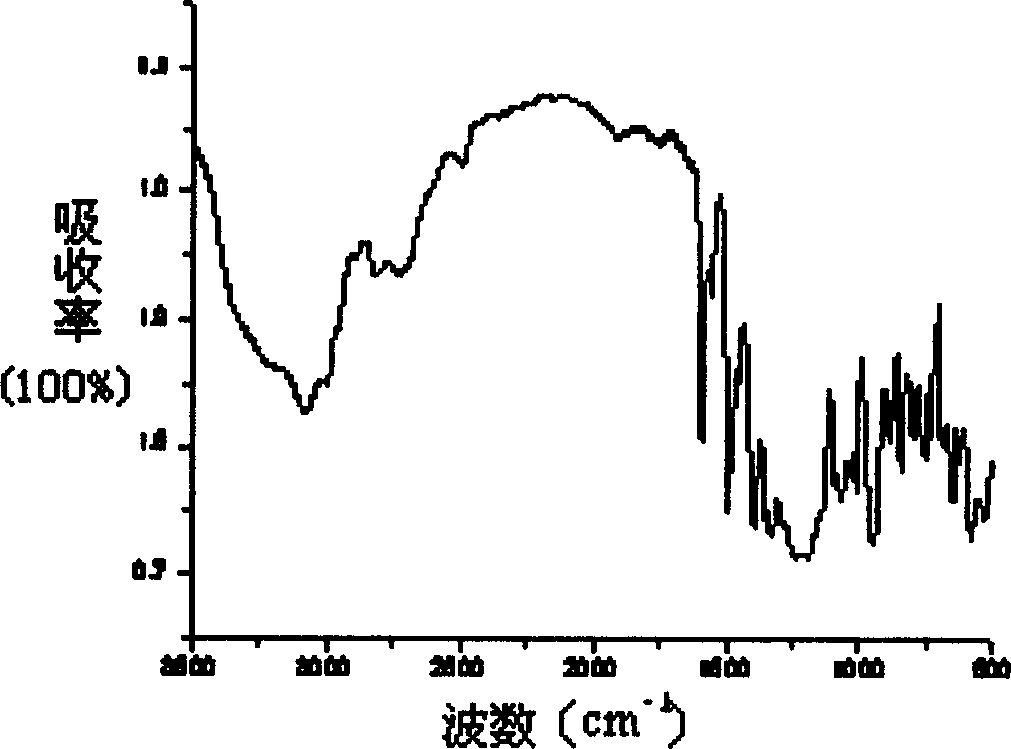
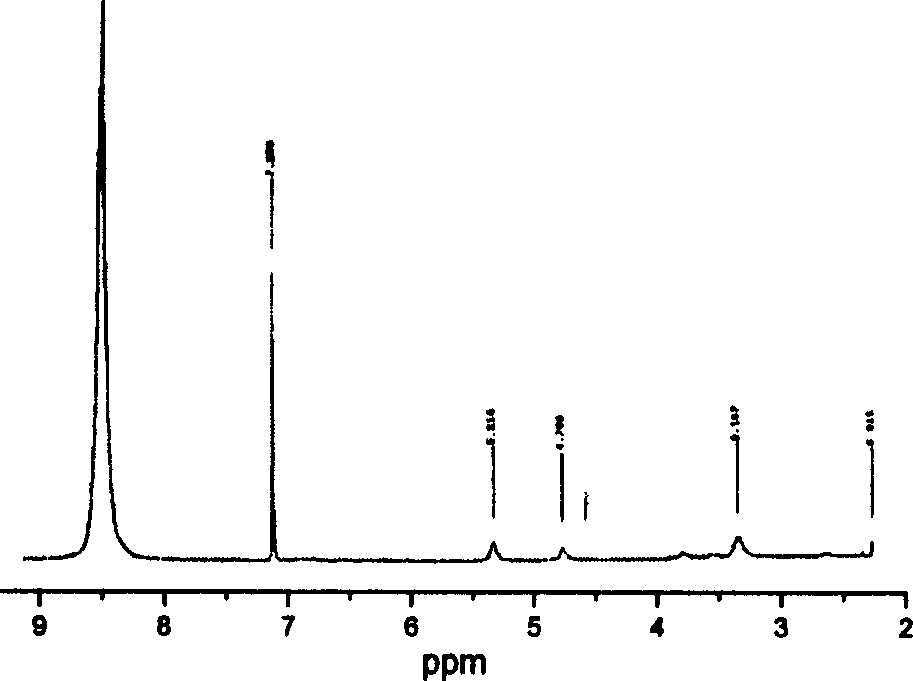
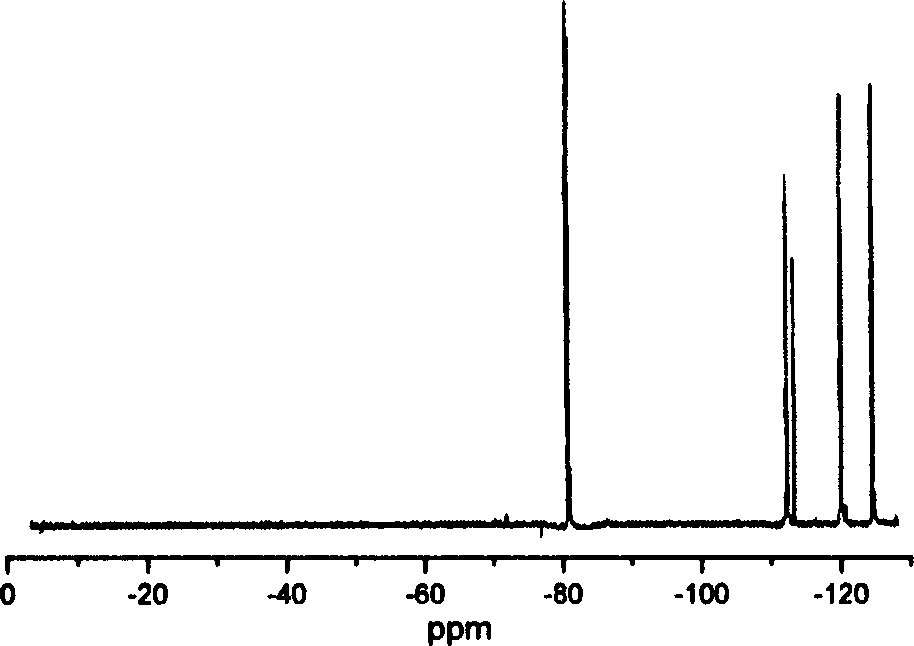
[0039] Structural characterization: The results of SEM test show that the...
Embodiment 3
[0040] Example 3: Synthesis of polystyrene peralkylfluorosulfonimides containing alkyl side chains
[0041] (1) Weigh 10g of uncrosslinked polystyrene containing side chains, add 10ml of 1,2-dichloroethane, then add 25g of chlorosulfonic acid, react at 60°C for 8 hours, and add 14ml of SOCl at the end of the reaction 2 After the reaction, the mixture was poured into ice water, the solid was washed to neutrality, and dried in vacuum to obtain a chlorosulfonated polymer.
[0042] (2) Weigh 5.0g of chlorosulfonated polymer, 7.5g of perfluorosulfonamide (C 8 f 17 SO 2 NH 2 ) by adding 25ml CH 2 ClCH 2 Cl, slowly add 7.5g of triethylamine dropwise, control the temperature at 60°C, and react for 40 hours. After the reaction, wash with dilute HCl, chloroform, and deionized water in sequence, extract and purify, and then dry under reduced pressure to obtain 11.6g of the product .
[0043] Structural characterization: The results of SEM test show that the porous structure of per...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com