Catalytic preparation of beta-nicotinamide mononucleotide by immobilized whole-cell one-step enzymatic reaction
A technology for the catalytic preparation of nicotinamide, applied in the field of molecular biology and biology, to achieve the effect of high technical difficulty, less coenzyme consumption, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
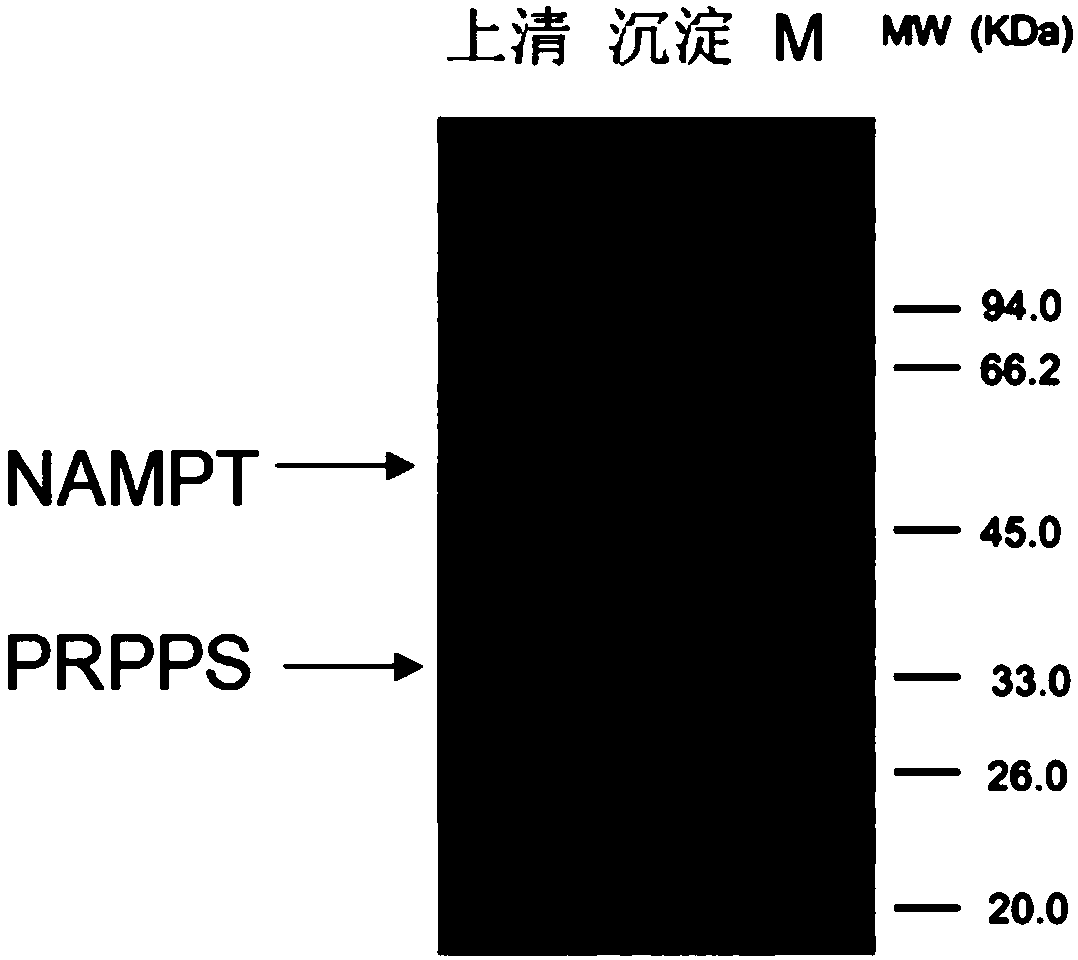
[0023] The construction of embodiment 1 enzyme gene co-expression system
[0024] The NAMPT gene was constructed on the plasmid pET-42a by using the restriction enzyme cutting sites NdeI and EcoRI to form the recombinant plasmid pET-42a-NAMPT. Then use the primers with SacI and XhoI to amplify the gene PRPPs, use the restriction enzymes FDSacI and FD XhoI to quickly cut the amplified fragment of PRPPs and the recombinant plasmid pET-42a-NAMPT, and under the action of the ligase NEBligase, PRPPs and The two genes of NAMPT were cloned into pET-42a at the same time to form a new recombinant whole gene plasmid pET-42a-NAMPT-PRPPs. The recombined whole-gene plasmid was heat-shocked in a water bath at 42°C for 90s and transferred into competent cells BL21(DE3). The transformation solution was incubated in a constant temperature shaking box at 37°C for 1h, and then spread on a plate containing 100mg / L kanamycin, cultured at 37°C for 24h, and single clones were picked. Randomly scre...
Embodiment 2
[0025] Embodiment 2 one-step enzymatic method prepares β-NMN
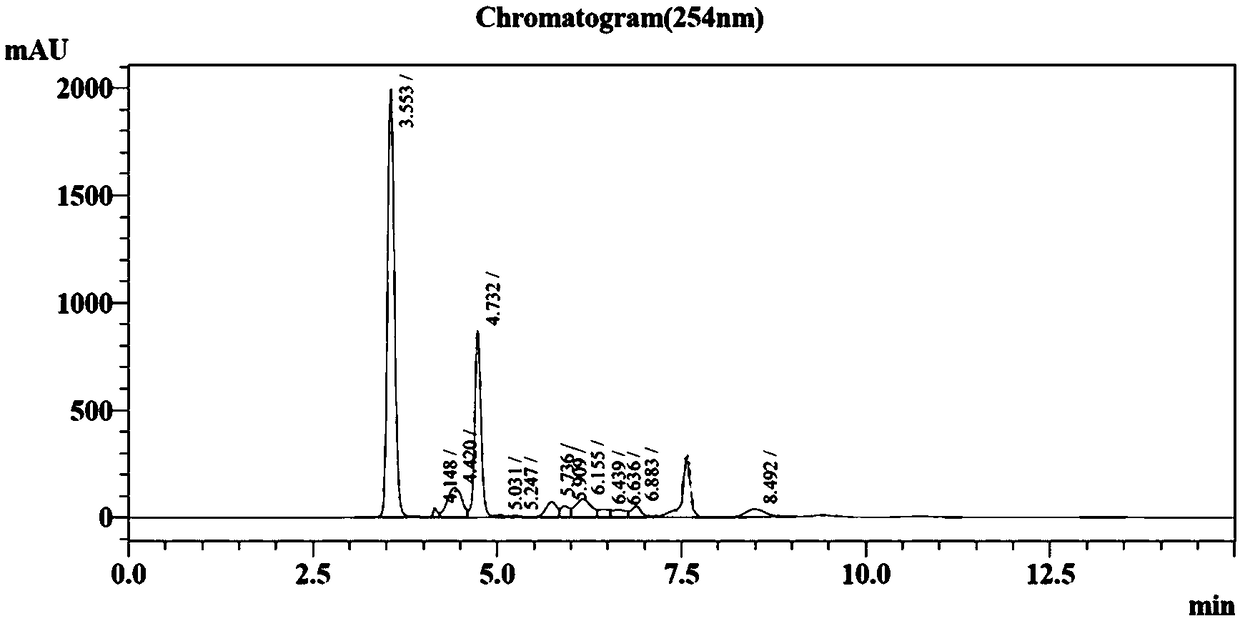
[0026] In order to reduce the amount of bacteria used in the reaction system and make full use of the high-density expression of proteins in microbial cells, the same bacterial cell was developed to express two enzymes. The specific transformation reaction system contains 20mM nicotinamide, 20mM ATP, 40mM MD-5-phosphate ribose, 10mM MgCl 2 , 20mM MnSO 4 , and adjust the pH to 8.0 with saturated sodium hydroxide. Then, 10 g / L of bacterial cells co-expressing PRPPs and NAMPT were dropped into the raw material solution, and stirred while adding to ensure that the bacterial cells were fully dissolved, the stirring speed was controlled at 60 rpm, and the temperature was at 37° C. 15% K 2 CO 3 The pH of the solution was controlled at 8.3, and a sample was taken for HPLC analysis after reacting for 3 hours. By comparing with the standard, the amount of β-NMN produced was 5.8 g / L, and the conversion rate was 86.8%.
Embodiment 3
[0027] Example 3 One-step enzymatic preparation of β-NMN
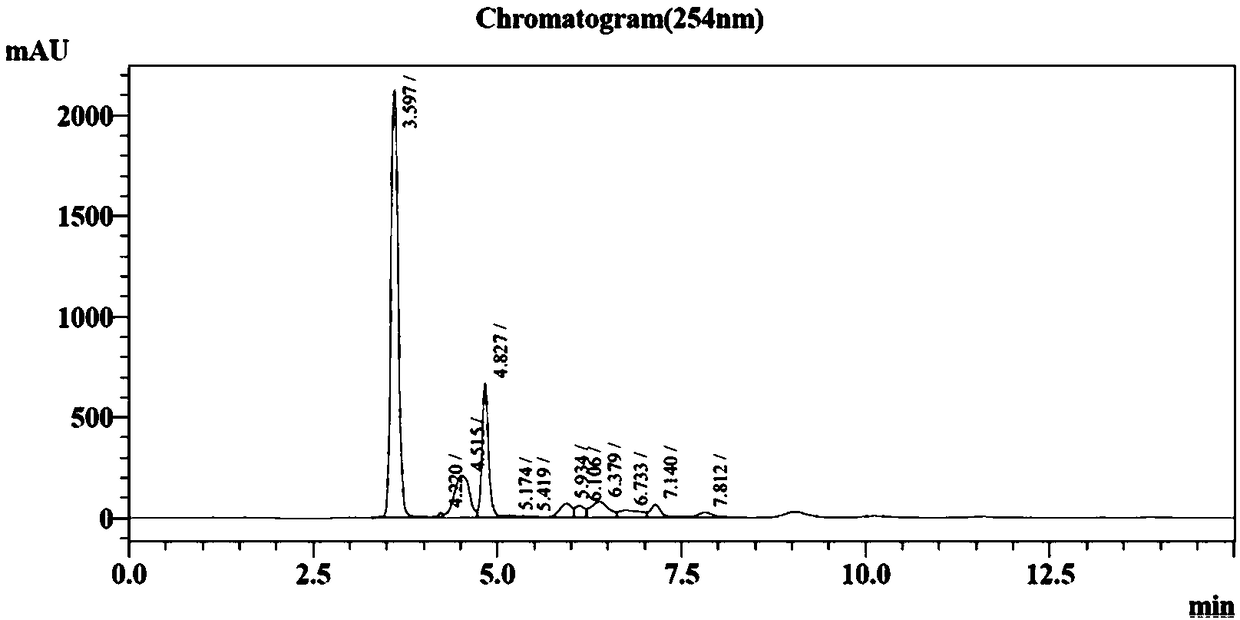
[0028] A transformation system containing 50mM nicotinamide, 40mM ATP, 80mM D-5-phosphate ribose, 10mM MgCl 2 , 20mMMnSO 4 , and adjust the pH to 8.3 with saturated sodium hydroxide. Then, 20 g / L of bacterial cells co-expressing PRPPs and NAMPT are dropped into the raw material solution, and stirred while adding to ensure that the bacterial cells are fully dissolved, the stirring speed is controlled at 60 rpm, and the temperature is at 37° C. 15% K 2 CO 3 Solution control pH is at 8.3, takes a sample after reacting 3h and carries out HPLC analysis, by contrasting with standard substance, can obtain the β-NMN generation amount of 12g / L (see figure 2 ), conversion rate is at 89.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com