Sustained release drug nanofibers and preparation method thereof
A technology of nanofibers and slow-release drugs, which is applied in the direction of pharmaceutical formulations, drug delivery, and medical preparations of non-active ingredients. It can solve problems such as unsatisfactory drugs, and achieve improved encapsulation efficiency, maintenance of biological activity, and long-term stable release. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
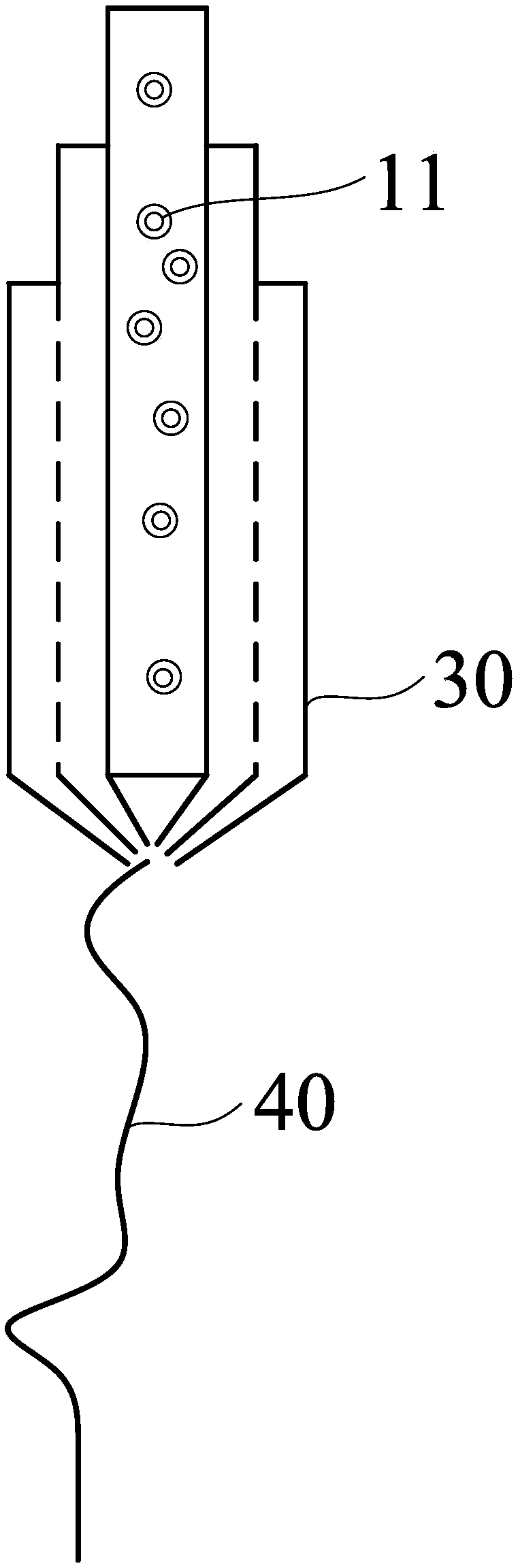
[0045] see figure 2 , the embodiment of the present invention also provides a preparation method of the sustained-release drug nanofibers, comprising the following steps:
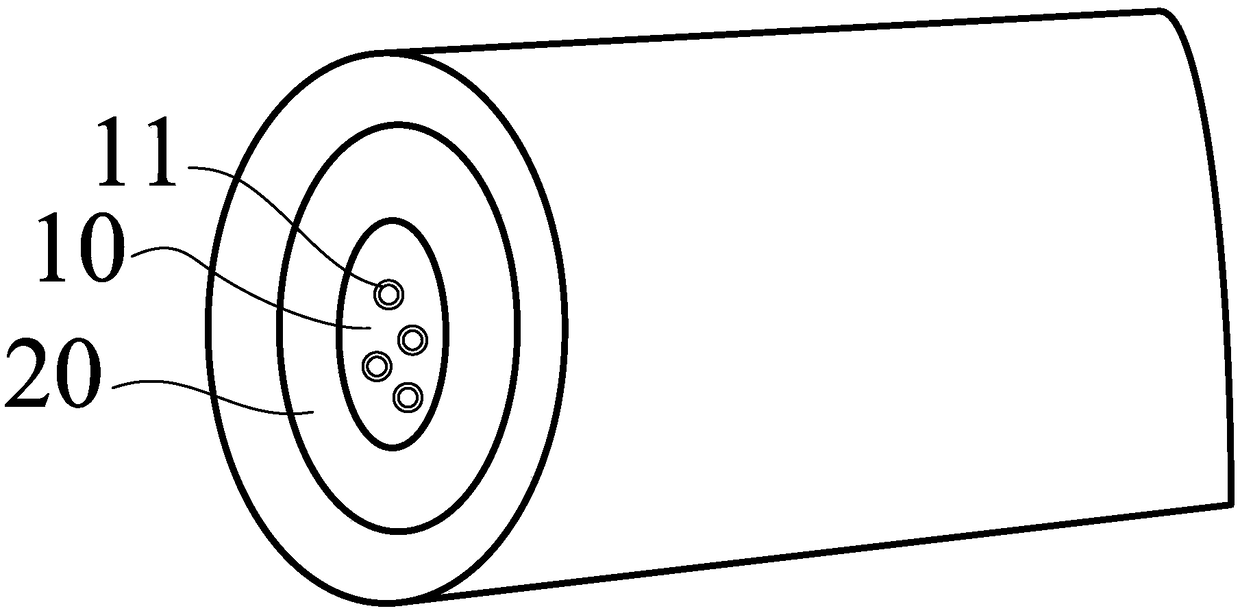
[0046] S100, providing the drug-loaded nanospheres 11;
[0047] S200, mixing the drug-loaded nano-microspheres 11 and the core sustained-release material in a solvent to obtain a core spinning solution;
[0048] S300, prepare a plurality of shell spinning solutions respectively, each shell spinning solution includes a solution of the material of the nanofiber slow-release layer 20;
[0049] S400, performing coaxial electrospinning on the core layer spinning solution and the plurality of shell layer spinning solutions with a coaxial spinning needle, and the coaxial spinning needle has a plurality of coaxially arranged liquid outlets , for simultaneously outputting the core layer spinning solution and the plurality of shell layer spinning solutions respectively.
[0050] The drug-loaded nano-microspheres ...
Embodiment 1
[0063] Example 1 Preparation of drug-loaded nanospheres 11
[0064] This embodiment is used to prepare drug-loaded nanospheres 11 with two layers of sustained-release layers of nanospheres. The sustained-release layers of nanospheres are chitin layer and BSA layer respectively.
[0065] Mix 80 μg of BMP-2 and 100 mg of BSA, and dissolve with 10 mL of deionized water under slight magnetic stirring to obtain a drug-loaded BSA aqueous solution;
[0066] Add 50mg of chitin to 50mL of 0.5wt% acetic acid solution, stir magnetically, and prepare a 1mg / mL chitin solution;
[0067] Under the condition of magnetic stirring, a total of 40 mL of ethanol was uniformly injected into the drug-loaded BSA aqueous solution in the first step at a rate of 2 mL / h with a micro-injection pump, and then stirred overnight;
[0068] Under the condition of magnetic stirring, inject 40 mL of the chitin solution prepared in the second step at a constant speed of 0.5 mL / min with a micro-injection pump, an...
Embodiment 2
[0071] Example 2 Preparation of drug-loaded nanospheres 11
[0072] The difference between this example and Example 1 is that the sustained-release layer of the drug-loaded nanospheres 11 consists of three layers, namely a chitin layer, a BSA layer and a chitin layer.
[0073] Mix 80 μg of BMP-2 and 100 mg of BSA, and dissolve with 10 mL of deionized water under slight magnetic stirring to obtain a drug-loaded BSA aqueous solution;
[0074] Add 50mg of chitin to 50mL of 0.5wt% acetic acid solution, stir magnetically, and prepare a 1mg / mL chitin solution;
[0075] Under the condition of magnetic stirring, a total of 40 mL of ethanol was uniformly injected into the drug-loaded BSA aqueous solution in the first step at a rate of 2 mL / h with a micro-injection pump, and then stirred overnight;
[0076]Under the condition of magnetic stirring, inject 40 mL of the chitin solution at a constant speed of 0.5 mL / min with a micro-injection pump, and continue to stir for 8 hours to prepa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com