Method for producing aviadenovirus 4 type vaccine by using LMH cell line, and vaccine
A poultry adenovirus and cell line technology, applied in the direction of microorganism-based methods, veterinary vaccines, biochemical equipment and methods, etc., can solve the problems of no obvious advantages and high production costs, and achieve the effect of improving immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, the preparation of LMH cell
[0035] S1. LMH cells were cultured with cell culture medium containing 0.8% (v / v) newborn bovine serum, 100IU / ml penicillin and 0.25% (v / v) N-acetyl-D-glucosamine, 37°C, 5% CO 2 Culture until the cells grow to a density of 30-55%, and use 0.025% trypsin-EDTA (0.02%) to digest and disperse the cells;
[0036] S2. Culture the LMH cells obtained in step S1 with a cell culture solution containing 0.8% (v / v) neonatal bovine serum, 100 IU / ml penicillin and 1.25% (v / v) N-acetyl-D-glucosamine, 37 °C, 5% CO 2 Culture until the cells grow to a density of 60-75%, and use 0.025% trypsin-EDTA (0.02%) to digest and disperse the cells;
[0037] S3, using the cell culture medium containing 0.8% (v / v) newborn calf serum, 100IU / ml penicillin and 2.5% (v / v) N-acetyl-D-glucosamine to culture the LMH cells obtained in step S2, 37 °C, 5% CO 2 Incubate until cells form a well-grown cell monolayer.
Embodiment 2
[0038] Embodiment 2, preparation of poultry adenovirus type 4 virus liquid
[0039] A) LMH cells were prepared according to the method described in Example 1;
[0040] B) Inoculate avian adenovirus type 4 into the LMH cells prepared in step A according to the virus inoculum MOT of 0.1, absorb the virus solution at 37°C for 60 minutes, and add 0.5% (v / v) newborn bovine serum and 100IU / ml penicillin cell maintenance solution, at 37°C, 5% CO 2 After culturing for 60 hours, when the cytopathic CPE reached more than 80%, the cytovenom was harvested.
[0041] C) The harvested cell venom was repeatedly frozen and thawed at -20°C for 3 times, centrifuged at 3000 rpm for 10 minutes, and the supernatant was collected to obtain a virus stock solution, which was concentrated 10 times by ultrafiltration to obtain a seedling virus solution;
[0042] D) the seedling virus solution obtained above is diluted serially by 10 times with DMEM culture fluid, and 10 -5 、10 -6 、10 -7 、10 -8 、10...
Embodiment 3
[0050] Embodiment 3, optimal virus harvesting time
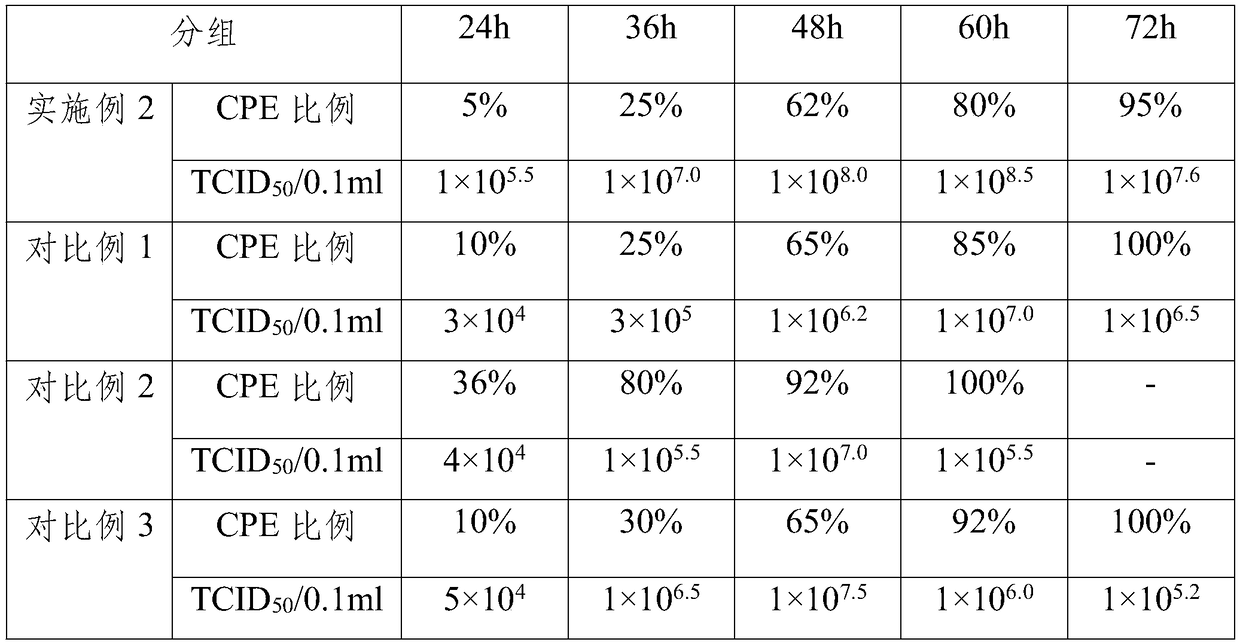
[0051] Observe the TCID of the virus liquid harvested at different times by preparing the poultry adenovirus type 4 vaccine preparation virus liquid by the method described in Example 2 and Comparative Examples 1 to 3 50 , and screen out the best virus harvesting time, the results are shown in Table 1 below.
[0052] Table 1 The TCID of the virus liquid harvested at different times 50
[0053]
[0054] As can be seen from Table 1 above, compared with Example 2, in Comparative Example 1, N-acetyl-D-glucosamine was not added to the LMH cell culture, and the LMH cells obtained from the prepared venom were used to prepare the seedling virus solution and reached the maximum in 60 h. value 10 7.0 TCID 50 / 0.1ml, the virus titer was significantly lower than in Example 2; in Comparative Example 2, the LMH cells obtained without setting the content of N-acetyl-D-glucosamine in a gradually increasing gradient were used to prepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com