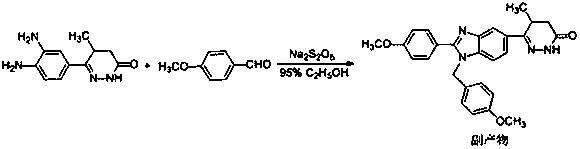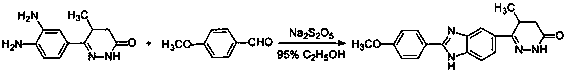Novel method for preparing pimobendan from by-product for synthesizing pimobendan
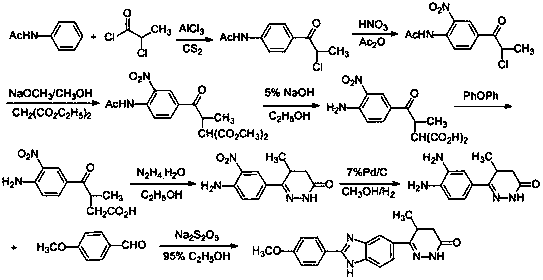
A technology of pimobendan and by-products, applied in the field of pharmaceutical chemical industry, can solve the problems of many reaction steps, unsuitable for industrialized production and the like, and achieve the effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
[0021] By-product (chemical name is (5RS)-6-[1-(4-methoxybenzyl)-2-(4-methoxybenzyl)-1 H -Benzimidazol-6-yl]-5-methyl-4,5-dihydro-3(2 H )-pyridazinone) (4.54 g, 10 mmol) was dissolved in a mixed solvent of 50 ml of acetone and 25 ml of water, cerium ammonium nitrate (10.96 g, 20 mmol) was added, and the reaction was stirred at 25°C for 2 hours, and a precipitate was precipitated, filtered , the precipitate was collected, and the obtained solid was washed successively with 100 ml of water and 20 ml of acetone, and dried to obtain 3.03 g of a white solid. Add 45 ml of ethyl acetate to the resulting white solid, stir, heat to reflux for 30 minutes, cool, and filter the resulting white precipitate to obtain 2.94 g of pimobendan, with a yield of 88.1%. m.p: 241.1~242.5℃. 1 H-NMR (500MHz, DMSO-d6) δ: 12.86 (br s, 0.5H), 12.83 (br s, 0.5H), 10.91 (br s, 0.5H), 10.88 (br s, 0.5H), 8.12 ( d, J = 9.0, 2H), 8.02 (br s, 0.5H), 7.85 (br s, 0.5H), 7.73 (d, J = 8.5Hz, 0.5H), 7...
Embodiment 2
[0023] By-product (chemical name is (5RS)-6-[1-(4-methoxybenzyl)-2-(4-methoxybenzyl)-1 H -Benzimidazol-6-yl]-5-methyl-4,5-dihydro-3(2 H )-pyridazinone) (9.08 g, 20 mmol) was dissolved in a mixed solvent of 100 ml of acetone and 50 ml of water, added cerium ammonium nitrate (32.9 g, 60 mmol), stirred at 22°C for 2.5 hours, precipitated, filtered , the precipitate was collected, and the obtained solid was washed successively with 200 milliliters of water and 40 milliliters of acetone, and dried to obtain 6.23 grams of a white solid. Add 90 ml of ethyl acetate to the resulting white solid, stir, heat to reflux for 30 minutes, cool, and filter the resulting white precipitate to obtain 6.01 g of pimobendan, with a yield of 90%.
Embodiment 3
[0025] By-product (chemical name is (5RS)-6-[1-(4-methoxybenzyl)-2-(4-methoxybenzyl)-1 H -Benzimidazol-6-yl]-5-methyl-4,5-dihydro-3(2 H )-pyridazinone) (4.54 g, 10 mmol) was dissolved in a mixed solvent of 50 ml of acetone and 25 ml of water, cerium ammonium nitrate (13.7 g, 25 mmol) was added, and the reaction was stirred at 20°C for 3 hours, a precipitate was precipitated, filtered , the precipitate was collected, and the obtained solid was washed successively with 100 ml of water and 20 ml of acetone, and dried to obtain 3.23 g of a white solid. Add 45 ml of ethyl acetate to the resulting white solid, stir, heat to reflux for 30 minutes, cool, and filter the resulting white precipitate to obtain 3.05 g of pimobendan, with a yield of 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com