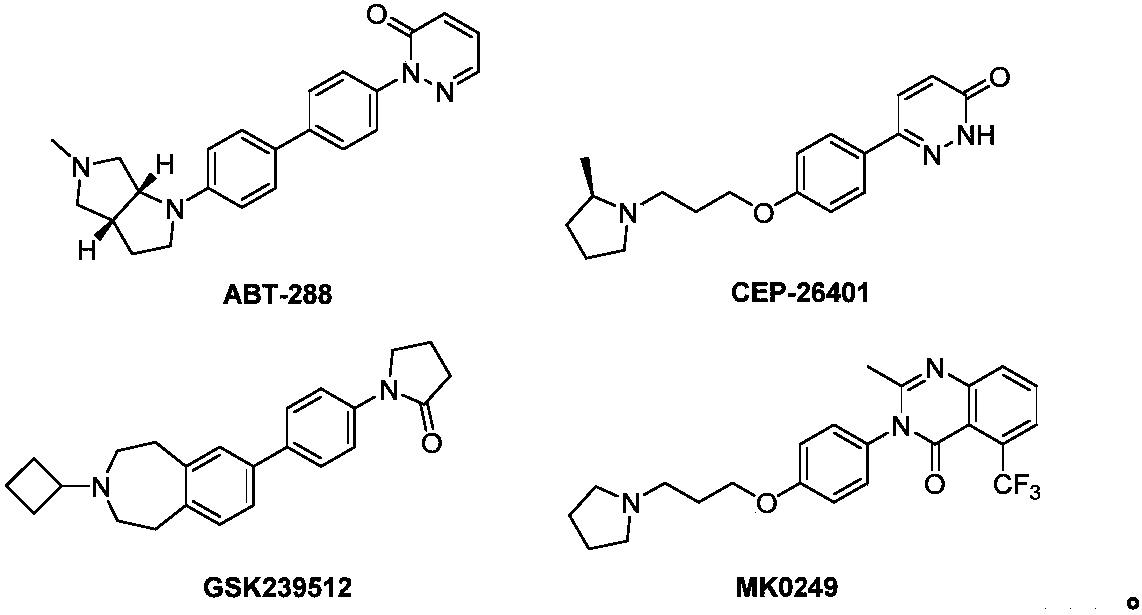
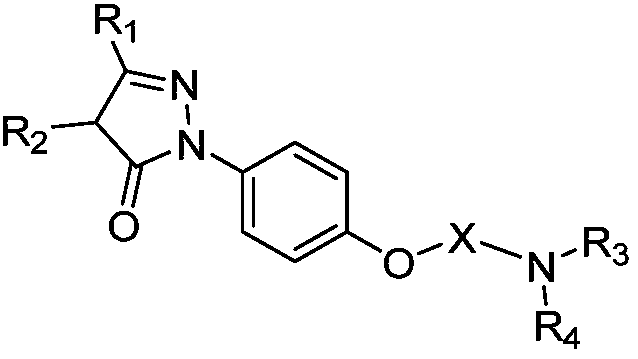
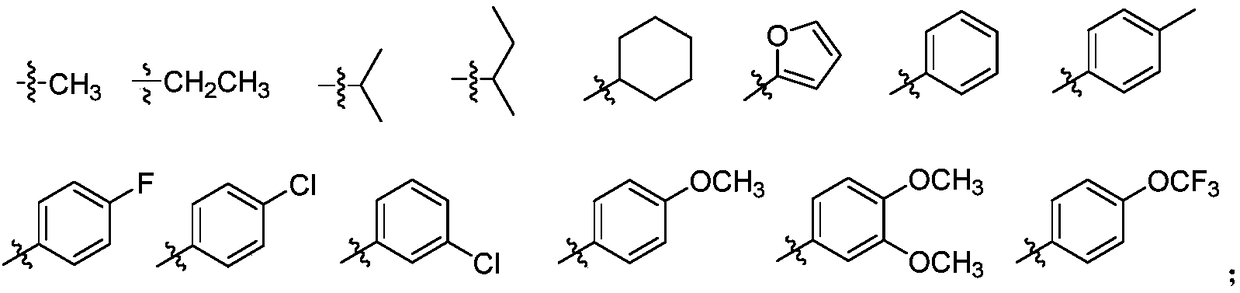
Substituted phenyl pyrazolone derivative as well as preparation method and application of substituted phenyl pyrazolone derivative
A pyrazoline and phenyl technology, applied in the field of medicine, can solve the problems of neurodegenerative diseases, such as complicated etiology, difficult drug treatment, and incomplete elucidation of pathogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 replaces the synthesis of ethyl acetoacetate
[0035] A Ethyl 2-methylacetoacetate (Intermediate 1)
[0036] Ethyl acetoacetate (1.3g, 0.1mol), CH 3 I (6.3mL, 0.1mol) and K 2 CO 3 (13.8g, 0.1mol), add 60mL CH 3 OH, reflux for 3h. After the reaction, the reaction solution was poured into 20 mL of cold water, concentrated under reduced pressure, and extracted three times with 50 mL of EtOAc. The organic layers were combined, washed with 0.5N HCl, washed with water, anhydrous Na 2 SO 4 It was dried and separated by column chromatography (petroleum ether: EtOAc=10:1) to obtain 10.1 g of a colorless liquid. Yield: 70%; ESI-MS: m / z=145[M+H] + .
[0037] B ethyl 2-phenylacetoacetate (intermediate 2)
[0038]Suspend NaH (326mg, 8.15mmol) in 15mL of anhydrous THF, add ethyl phenylacetate (1mL, 6.27mmol) dropwise under ice-cooling, and react at 60°C for 30min after the dropwise addition is complete. The reaction solution was cooled to 0°C, and 2 mL of a TH...
Embodiment 2
[0059] Embodiment 2 Synthesis of 1-phenylpyrazolone intermediate
[0060] A 3,4-Dimethyl-1-(4-benzyloxyphenyl)-2-pyrazolin-5-one (intermediate 13)
[0061] Weigh 500mg 1-benzyloxy-4-phenylhydrazine hydrochloride (2mmol), add to 10mL H 2 O, stirred for 30 min, added an equimolar amount of NaOH 80 mg, and stirred for 30 min. Add ethyl 2-methylacetoacetate (247mg, 1.9mmol) dropwise to the above reaction solution, heat up to reflux for 2.5h, stop heating, cool to room temperature, filter, and filter residue with petroleum ether: EtOAc (5:1) for pulping , Petroleum ether: EtOAc (3:1), washed and dried to give 350 mg of light yellow solid. Yield: 63%; 1 H NMR (500MHz, CDCl 3 ): δ7.79(d, J=9.0Hz, 2H), 7.46-7.33(m, 5H), 7.02(d, J=9.0Hz, 2H), 5.10(s, 2H), 3.23(q, J= 8.0Hz, 1H), 1.46(d, J=8.0Hz, 3H), 1.28(s, 3H); ESI-MS: m / z=295[M+H] + .
[0062] B 3-methyl-4-phenyl-1-(4-benzyloxyphenyl)-2-pyrazolin-5-one (intermediate 14)
[0063] The preparation method is the same as that of i...
Embodiment 3
[0088] The synthesis of embodiment 3 hydrogenolysis products
[0089] A 4,5-Dimethyl-2-(4-hydroxyphenyl)-3-pyrazolone (Intermediate 27)
[0090] Intermediate 13 (820 mg, 2.79 mmol) was dissolved in 20 mL CH 3 160 mg of 10% Pd / C was added to OH, and reacted overnight at room temperature under hydrogen pressure. After the reaction, Pd / C was removed by filtration, and the filtrate was concentrated to obtain 460 mg of light yellow solid, which was washed with a small amount of ice EtOAc. Yield: 81%; 1 H NMR (400MHz, DMSO-d 6 ):δ10.33(s,1H),9.42(s,1H),7.43(d,J=8.8Hz,2H),6.80(d,J=8.8Hz,2H),2.05(s,3H),1.75 (s,3H); ESI-MS: m / z=205[M+H] + .
[0091] B 5-methyl-4-phenyl-2-(4-hydroxyphenyl)-3-pyrazolone (intermediate 28)
[0092] The preparation method is the same as that of intermediate 27, and intermediate 14 is used instead of intermediate 13 to obtain a pale yellow solid. Yield: 71%, 1 HNMR (400MHz, DMSO-d 6 ): δ9.53(s, 1H), 7.57-7.52(m, 2H), 7.48(d, J=8.8Hz, 2H), 7.41(t, J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com