Analogue obtained by disulfide bond cyclization of opium and neuropeptide FF receptor multi-target molecule BN-9 as well as preparation method and application thereof
A BN-9, neuropeptide technology, applied in the field of biochemistry, can solve the problems of low effective analgesic dose, maternal intolerant, long analgesic effect time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The synthetic method of embodiment 1 compound 1-8:
[0082] Experimental reagents: the resin is Rink-Amide-MBHA-Resin (substitution value S is 0.43mmol / g; Tianjin Nankai Hecheng Company), O-benzotriazole-N, N, N', N'-tetramethyl Urea-hexafluorophosphate (HBTU) (Shanghai Gil Biochemical Co., Ltd.), N-hydroxybenzotriazole (HOBt) (Shanghai Gil Biochemical Co., Ltd.), N, N-diisopropylethylamine (DIEA) ( Beijing Bailingwei), 1,8-diazabicycloundec-7-ene (DBU; Bailingwei). Ninhydrin is a product of Shanghai Reagent No.3 Factory. Dichloromethane (DCM), N,N-dimethylformamide (DMF), hexahydropyridine (piperidine), methanol (MeOH) and pyridine were all purchased from Tianjin Second Reagent Factory, trifluoroacetic acid (TFA), Phenol and pyridine are both products of Tianjin Reagent No. 1 Factory; the above organic reagents were redistilled before use.
[0083] Experimental equipment: solid-phase peptide synthesizer (designed by our laboratory), rotary evaporator (RE-5298A, Shan...
Embodiment 2
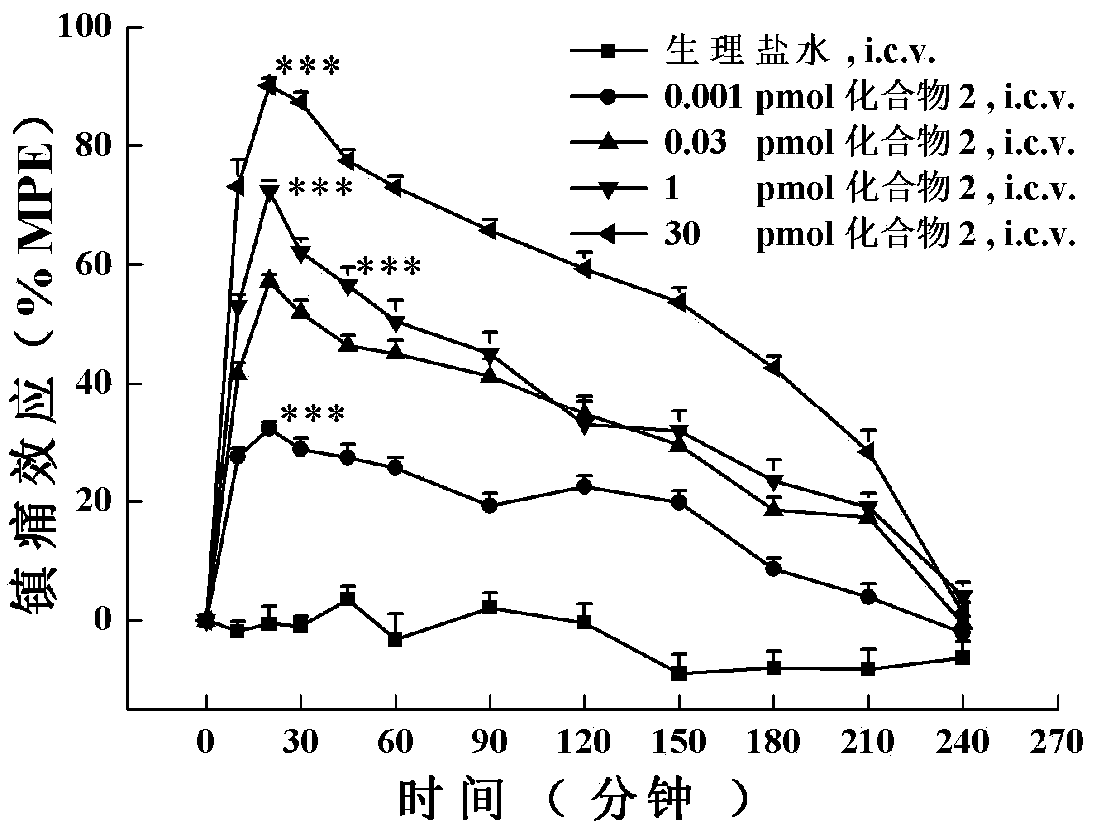
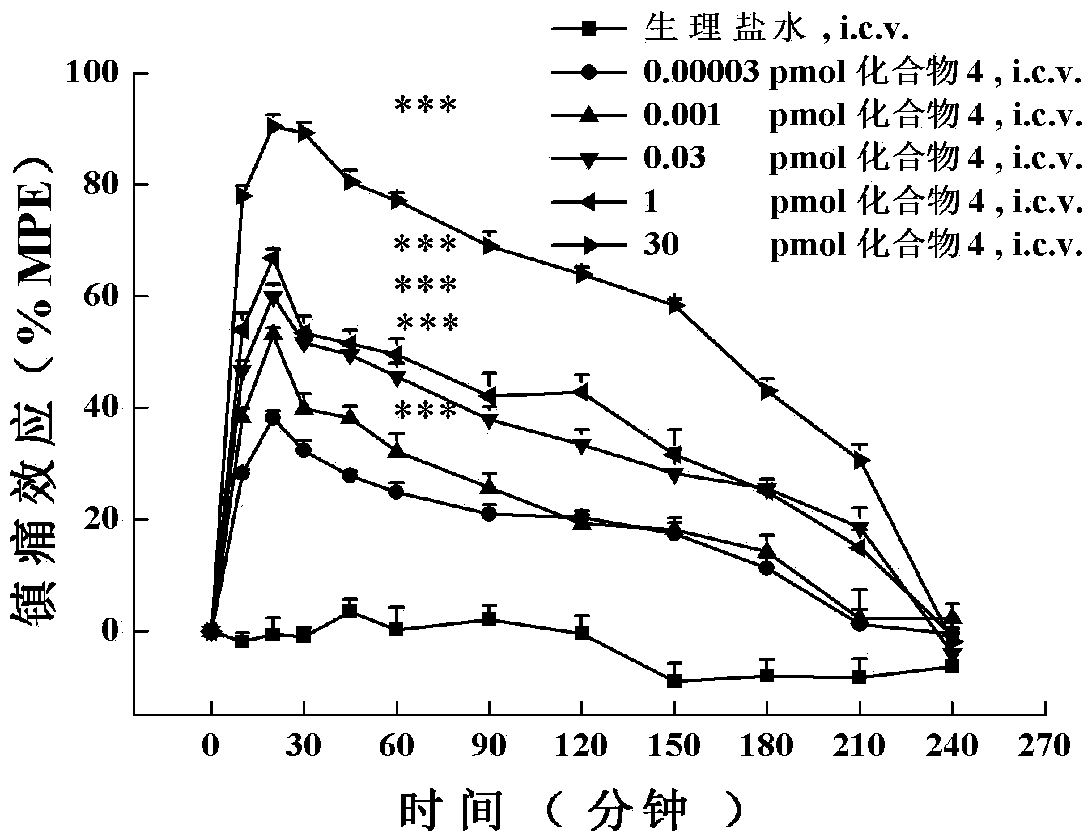
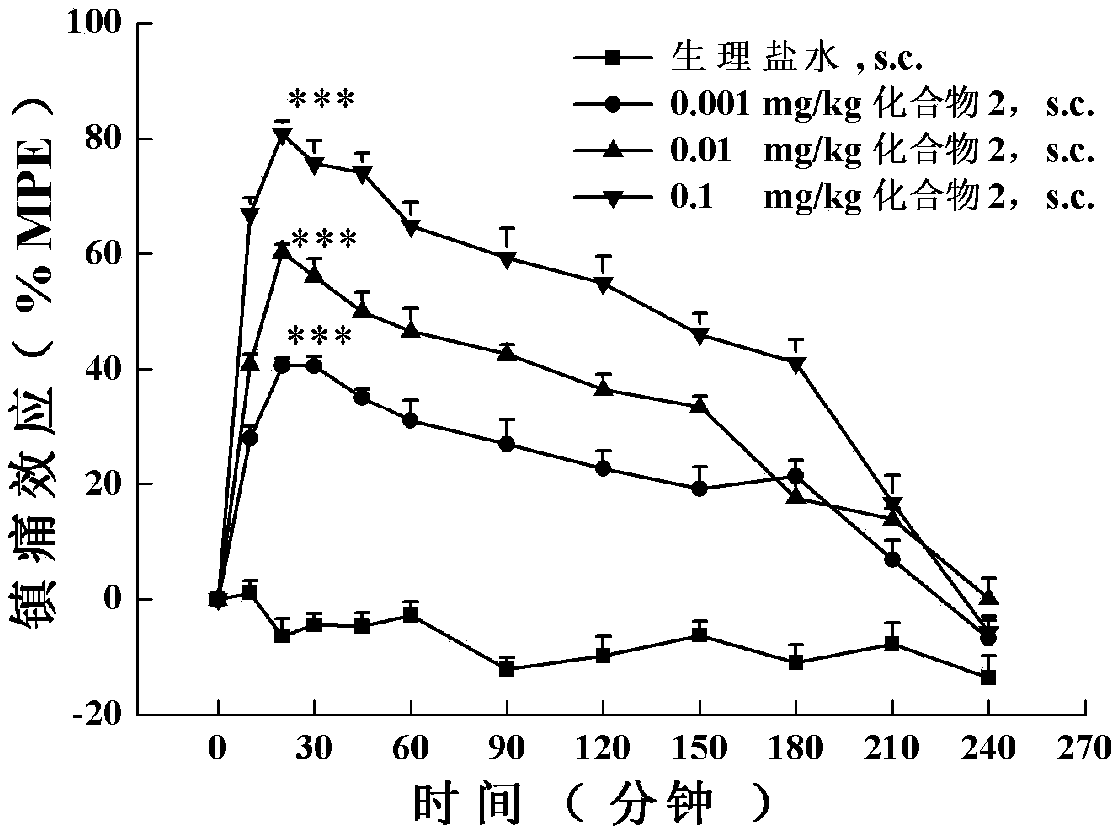
[0169] The in vitro functional activity assay of embodiment 2 analogs to opioid and NPFF receptors:
[0170] In stable expression of Mu-, Delta-, Kappa-opioid and NPFF 1 and NPFF 2 In HEK293 cells with receptors, their effects on these five receptors were detected by examining the regulation of the multi-target cyclized polypeptide of the opioid and NPFF receptors on Forskolin-induced intracellular cyclic adenosine monophosphate (cAMP) accumulation. agonistic activity. The specific method is: stably expressing Mu-, Delta-, Kappa-opioid and NPFF 1 and NPFF 2 Recipient HEK293 cells were seeded in 24-well plates at 120,000 cells per well, and cultured in an incubator for more than 20 hours. At the beginning of the experiment, the cell culture medium was replaced with preheated serum-free medium containing 1 mM IBMX, and incubated at 37°C for 10 min. Then, 10 μl of the drug to be tested and 10 μM forskolin (final concentration) were added to each well, and incubated at 37° ...
Embodiment 3
[0177] The in vitro enzymolysis stability measurement of embodiment 3 analogs:
[0178] Enzymatic Stability Test, an in vitro test to identify the stability of drugs in brain homogenate or plasma. Following incubation of compounds with brain homogenates or plasma, samples were analyzed for concentration by reverse phase HPLC. The half-life of the compound is then calculated. The specific method of enzymatic stability test is as follows:
[0179] 30±5g adult male mice were sacrificed by cervical dislocation, and the brain was removed (cerebellum and pons were removed), washed several times with ice-cold 1mM Tris-HCl buffer solution (pH=7.4) to remove residual blood on the brain, and blotted dry with filter paper , weighing. Add 50 times the volume of ice-cold 1 mM Tris-HCl (pH=7.4) (w / v=2%) buffer, place on ice for homogenization. The homogenate was placed in an ice bath for 30 minutes to facilitate cell lysis. Then add 0.5ml of 50mM ice-cold Tris-HCl for every 1ml of ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com