Human thyrotropin receptor antibody chemiluminescence detection kit as well as preparation method and application method thereof
A technology for chemiluminescent detection and thyrotropin detection, which is applied in the field of immunoassay medical detection, can solve the problems of inability to distinguish stimulatory, inhibitory, and neutralizing antibodies of human thyrotropin receptors, poor detection sensitivity, and poor accuracy. Securing Sensitivity, Enhancing Specificity, and Improving Sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention also provides a preparation method of a human thyrotropin receptor antibody chemiluminescent detection kit, comprising the following steps:
[0043] 1) Preparation of calibrator with human thyrotropin stimulating antibody in human serum matrix;
[0044] 2) Preparation of quality control products with human thyrotropin-stimulating antibodies in human serum matrix;
[0045] 3) Preparation of R1 reagent
[0046] After diluting the streptavidin magnetic beads with the magnetic bead dilution buffer, prepare a streptavidin-coated magnetic bead solution with a magnetic bead concentration of 0.05%-0.1%, which is the R1 reagent;
[0047] 4) Preparation of R2 reagent
[0048] Use a 50KDa pore size ultrafiltration centrifuge tube to replace the thyrotropin receptor stimulating antibody buffer with 50mM-200mM PBS buffer, add acridinium ester, mix well, and place it at room temperature for 2-4 hours, then purify the marker with AKTA protein purifier Antibody...
Embodiment 1
[0054] Embodiment 1 prepares TRAb chemiluminescent detection kit of the present invention
[0055] 1. Preparation of calibrator
[0056] Dilute TRAb into calibrators with 100mM PBS buffer containing 20% human serum, aliquot into 0mIU / mL, 0.3mIU / mL, 2.5mIU / mL, 5mIU / mL, 10mIU / mL, 20mIU / mL, 40mIU / mL .
[0057] 2. Preparation of Quality Control Products
[0058] The TRAb antibody was diluted with 100mM PBS buffer solution containing 20% human serum to form a quality control product, and aliquoted into two-point quality control products with a concentration of 1mIU / mL and 25mIU / mL.
[0059] 3. Preparation of R1 reagent in magnetic bead solution coated with streptavidin
[0060] Dilute the streptavidin magnetic beads with magnetic bead dilution buffer to a concentration of 0.1%, and store at 4°C.
[0061] 4. Preparation of acridinium ester-labeled thyrotropin receptor stimulating antibody R2 reagent
[0062] Add 500 μg of thyrotropin receptor stimulating antibody into a 50K...
Embodiment 2
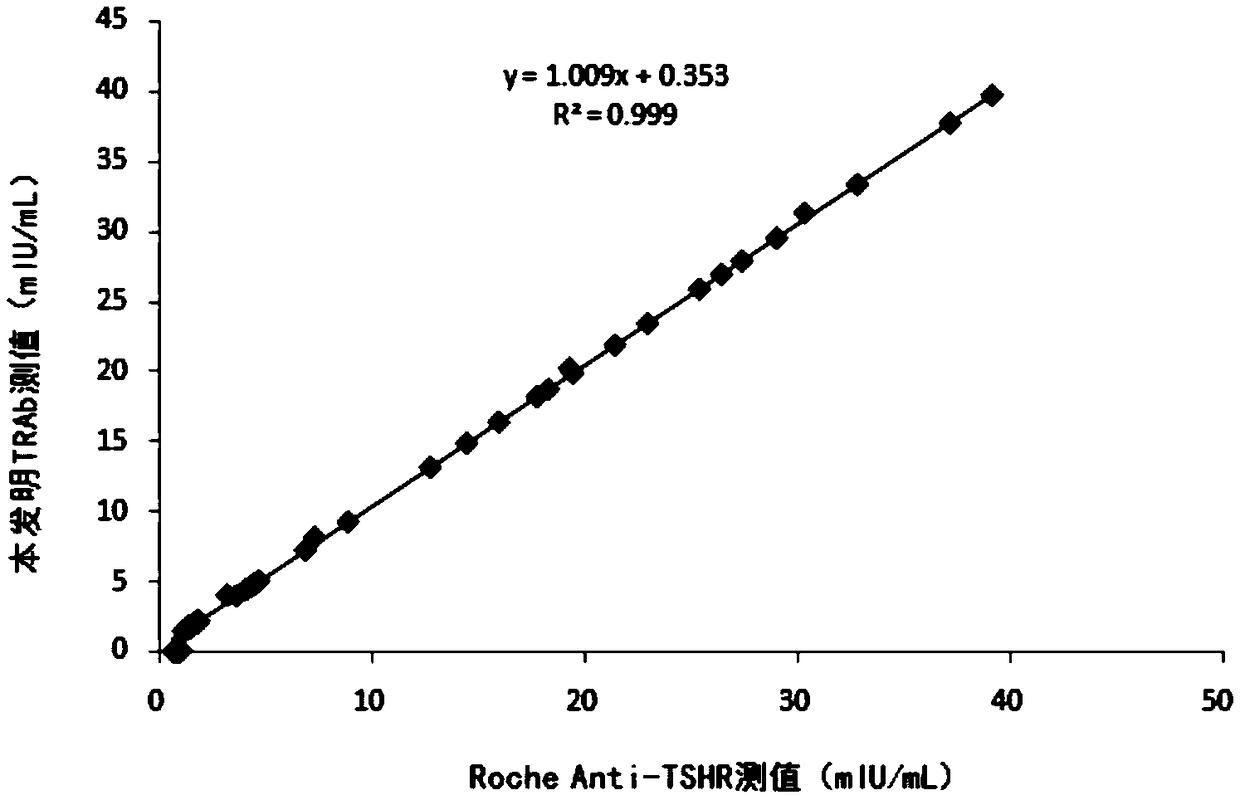
[0072] Embodiment 2 The methodological examination of TRAb chemiluminescence detection kit of the present invention
[0073] The test kit prepared in Example 1 is tested according to industry standards and testing procedures in the art, and the results are as follows:
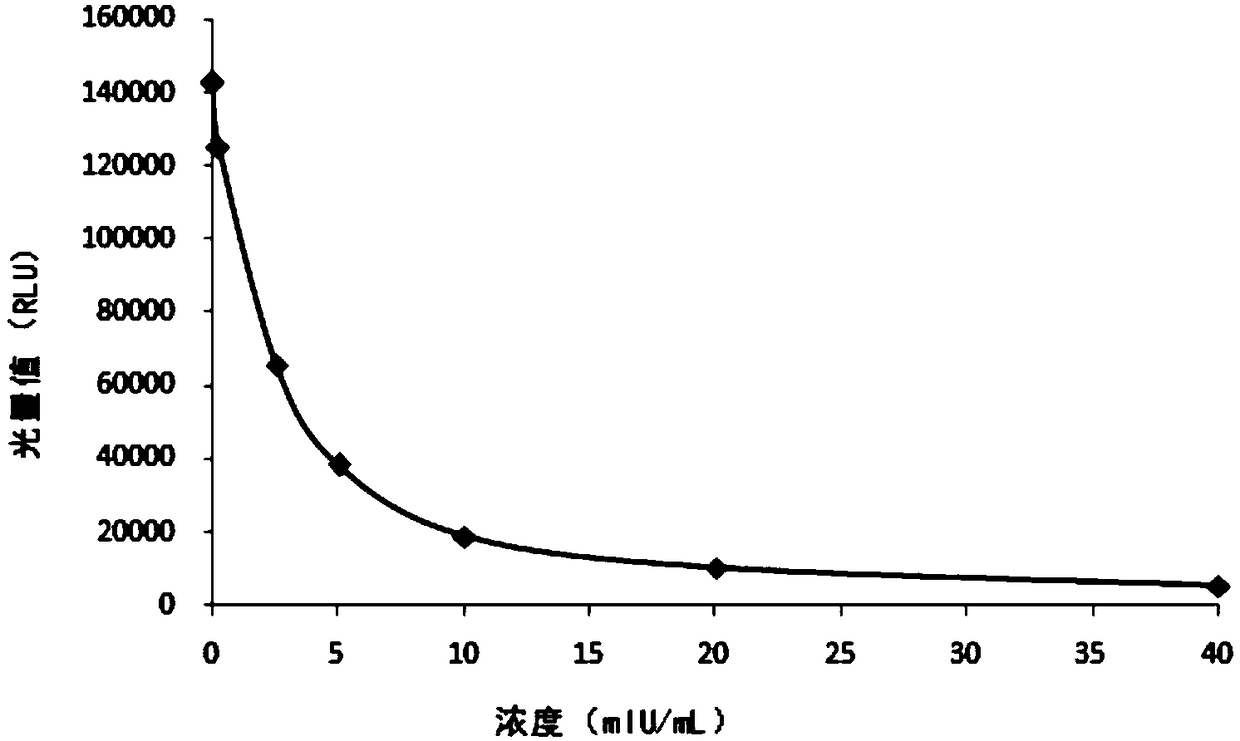
[0074] 1. Determination of the linear range of the kit
[0075] One batch of the kit prepared in Example 1 was used to measure the linear range samples 0mIU / mL, 0.3mIU / mL, 2.5mIU / mL, 5mIU / mL, 10mIU / mL, 20mIU / mL, 40mIU / mL, and the linear The range is 0.3-40mIU / mL, the correlation coefficient R2=0.9999.
[0076] Table 1 Determination of the linear range of the kit
[0077] Determination of calibrator concentration (mIU / mL)
Relative Luminous Intensity (RLU)
0
142648
0.3
125453
2.5
65633
5
38415
10
18752
20
10247
40
5410
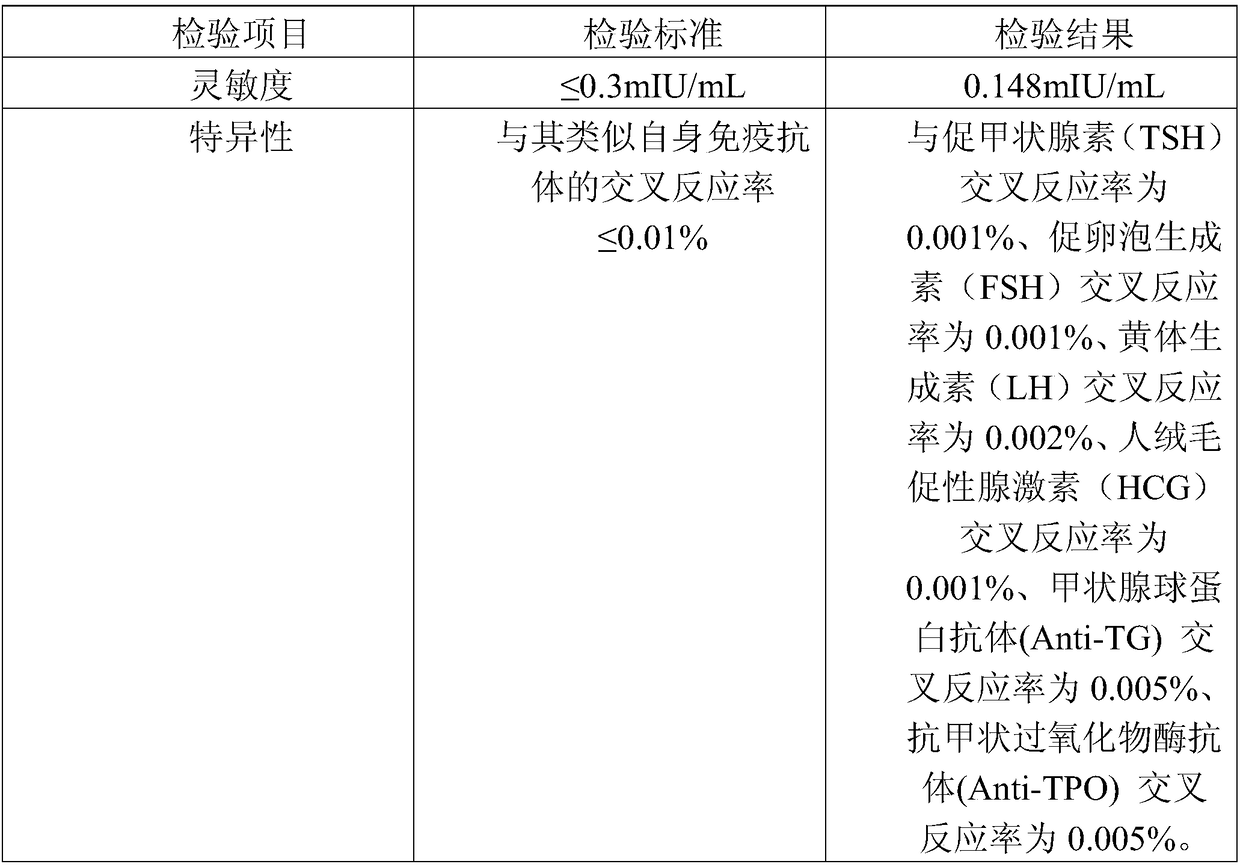
[0078] 2. Determination of Kit Sensitivity
[0079] With one batch of the kit prepared in Example 1, measure 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com