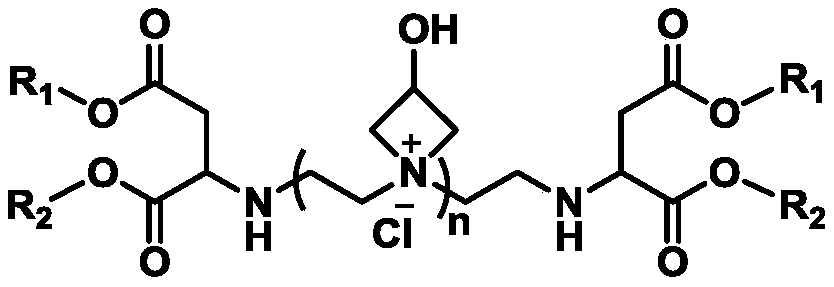
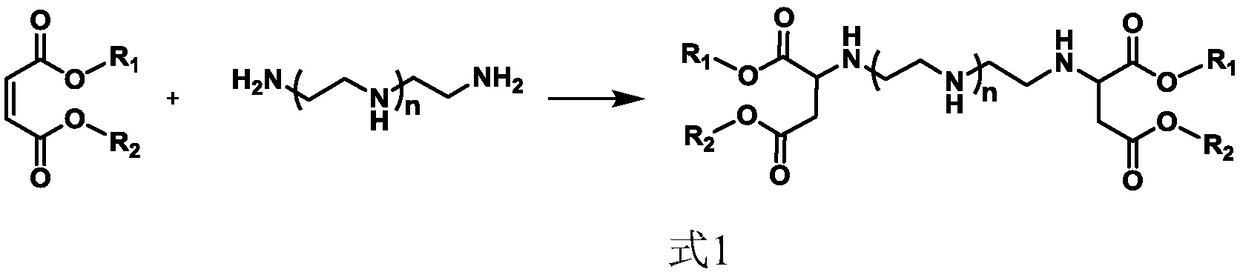
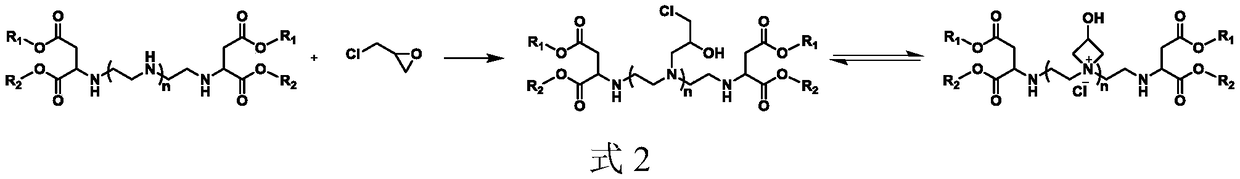
Water-soluble cationic polyaspartic ester, preparation method and application thereof
A technology of aspartic acid ester and water-soluble cation, which is applied in polyurea/polyurethane coatings, coatings, organic chemistry, etc., can solve the problems that it is difficult to meet the various needs of water-based polyurea, achieve good stability, and improve mechanical properties. The effect of intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Add 103.17g (1mol) of diethylenetriamine into a 1L four-neck round bottom flask equipped with a mechanical stirring paddle, a thermometer, a constant pressure dropping funnel, and a N 2 Air line pipe, bubbler, add 344.36g (2mol) diethyl maleate into the constant pressure dropping funnel, pass N into the system 2 For 10 minutes, replace the air in the system; slowly drop diethyl maleate into the flask with stirring at 25°C, drop it at a constant speed for 1 hour, control the reaction temperature to 30°C, then raise the temperature to 60°C for 3 hours, and stop the reaction.
[0044] Add 380g of distilled water to the above system, increase the stirring speed to 800rpm, emulsify the system, then reduce the speed to 400rpm, add 92.52g (1mol) epichlorohydrin into the constant pressure dropping funnel, slowly drop into the emulsion, 1h After dripping, the temperature was raised to 50°C to keep the temperature for reaction. After 50 minutes of reaction, the emulsion became c...
Embodiment 2
[0051] Add 103.17g (1mol) of diethylenetriamine into a 1L four-neck round bottom flask equipped with a mechanical stirring paddle, a thermometer, a constant pressure dropping funnel, and a N 2 Air line pipe, bubbler, add 344.36g (2mol) diethyl fumarate into the constant pressure dropping funnel, pass N into the system 2 For 30 minutes, replace the air in the system; slowly drop diethyl maleate into the flask under stirring at 25°C, drop it at a constant speed for 1 hour, control the reaction temperature to 30°C, then raise the temperature to 50°C for 6 hours, and stop the reaction.
[0052] Add 380g of distilled water to the above system, raise the stirring speed to 800rpm, emulsify the system, then reduce the speed to 400rpm, add 92.52g (1mol) epichlorohydrin in the constant pressure dropping funnel, slowly drop into the emulsion, 1.5 After dropping for h, the temperature was raised to 55°C and the temperature was kept for reaction. After 55 minutes of reaction time, the emu...
Embodiment 3
[0059] Add 116.99g (0.8mol) of triethylenetetramine into a 1L four-neck round bottom flask equipped with a mechanical stirring paddle, a thermometer, a constant pressure dropping funnel, and a N 2 Air pipe, bubbler, 275.49g (1.6mol) diethyl maleate was added to the constant pressure dropping funnel, and N was introduced into the system. 2 20min, replace the air in the system; slowly drop diethyl maleate into the flask under the condition of stirring at 25°C, drop it at a constant speed for 0.5h, control the reaction temperature at about 30°C, then raise the temperature to 40°C for 5h, stop the reaction .
[0060] Add 785g of distilled water to the above system, raise the stirring speed to 800rpm, emulsify the system, then reduce the speed to 400rpm, add 148.04g (1.6mol) epichlorohydrin into the constant pressure dropping funnel, slowly drop into the emulsion, After 1.5 hours of dripping, the temperature was raised to 55°C and the temperature was kept for reaction. After 50 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com