Heterocyclic aromatic compound substituted phosphine palladium sulfonate and nickel catalyst, ligand, preparation method and uses thereof
A technology for alkanes and alkenes, applied in the field of preparation, palladium phosphine sulfonate and nickel catalysts substituted by heterocyclic aromatic hydrocarbons and their ligands, which can solve the problem of low insertion ratio
- Summary
- Abstract
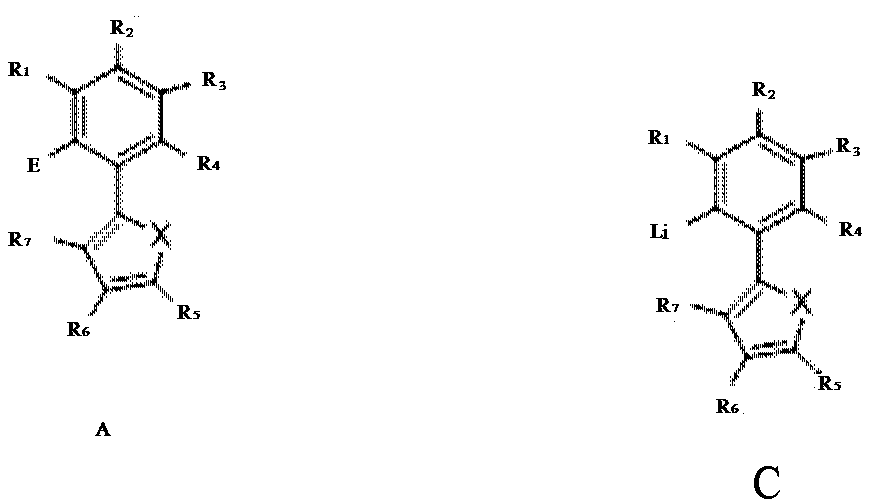
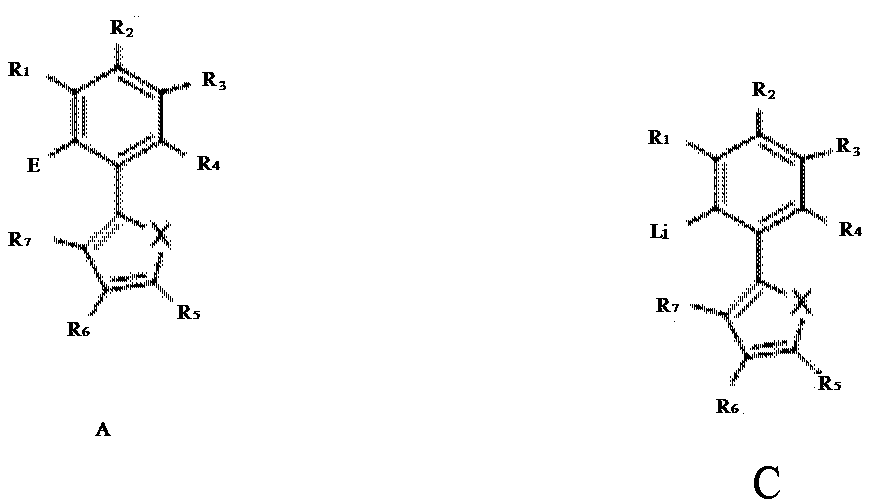
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
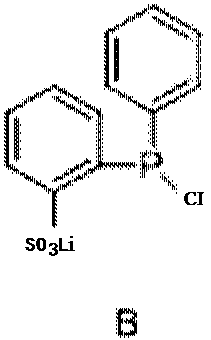
[0065] Example 1: 2-((2-furylphenyl)(phenyl)phosphino)benzenesulfonic acid
[0066]
[0067] Benzenesulfonic acid (1.58 g, 10 mmol) was dissolved in 25 ml of tetrahydrofuran solution, and 8 ml of n-butyllithium (concentration: 2.5 mol / L, 20 mmol) was slowly added dropwise at 0°C for 1 hour of reaction. Dissolve 10 mmoles of phenylphosphine dichloride in 25 ml of tetrahydrofuran solution and place it at minus 78°C, then slowly add tetrahydrofuran solution of lithium benzenesulfonate, and react at room temperature for 2 hours to obtain mixed solution A. 1-Bromo-2-furanbenzene (2.2 g, 10 mmol) was dissolved in 20 ml of tetrahydrofuran under nitrogen protection, cooled to minus 78°C, and 4 ml of n-butyllithium (concentration of 2.5 moles per liter, 10 millimoles), and reacted for 1 hour at minus 78°C. The mixed solution A was added thereto at minus 78°C, and reacted at room temperature for 24 hours. The solvent was spin-dried, and the residue was dissolved in water. The aqueo...
Embodiment 2
[0068] Example 2: 2-((2-benzofurylphenyl)(phenyl)phosphino)benzenesulfonic acid
[0069]
[0070] The synthesis procedure was the same as above, and 1-bromo-2-benzofuranbenzene (2.72 g, 10 mmol) was added to obtain a white solid (3.3 g, 72% yield). 1H NMR (400MHz, CDCl3): δ 8.43(br, 1H), 7.97(br, 1H), 7.78(d, J=5.9Hz, 2H), 7.57-7.48(m, 8H), 7.37-7.35(d, J=7.6Hz 2H), 7.20-7.19(d, J=7.3Hz, 2H), 7.10(s, 2H).13C NMR(100MHz, DMSO) of L2: δ153.9(s), 151.8(s), 151.6(s), 138.3(d, JPC=13Hz), 137.2(s), 137.0(s), 135.0(d, JPC=11Hz), 134.3(s), 134.1(s), 133.8(s), 133.6( s), 133.4(s), 133.2(s), 129.6(s), 129.2(d, JPC=6Hz), 128.8(s), 128.6(s), 128.4(s), 128.3(s), 127.7(s ), 124.8(s), 123.1(s), 121.6(s), 111.2(s), 108.1(d, JPC=18Hz).31P NMR(162MHz, DMSO): δ-13.82.ESI-MS(m / z ): [M-H] - calculated for C26H18O4 PS, 457.0663; found: 457.0656.
Embodiment 3
[0071] Example 3: 2-((2-benzothiophenephenyl)(phenyl)phosphino)benzenesulfonic acid
[0072]
[0073] The synthesis procedure was the same as above, and 1-bromo-2-benzothiophenebenzene (2.88 g, 10 mmol) was added to obtain a white solid (3.8 g, 80% yield). 1H NMR (400MHz, CDCl3): δ 8.41(br, 1H), 7.77(br, 3H), 7.67(br, 3H), 7.56(br, 1H), 7.74-7.28(m, 9H), 7.12(br, 1H).13C NMR (100MHz, DMSO) of L3: δ151.76(d, J=26.3Hz), 149.62(d, J=7.1Hz), 142.27(d, J=4.8Hz), 140.81(d, J =4.1Hz), 140.03(s), 139.32(m), 138.48(m), 137.42(d, J=7.1Hz), 134.86(d, J=8.7Hz), 134.22 (d, J=11.4Hz), 133.48(dd, J=31.3, 9.8Hz), 132.78(d, J=9.7Hz), 132.20(t, J=27.7Hz), 131.41(d, J=8.5Hz), 130.79(s), 129.02(m ), 128.65(m), 128.35(d, J=5.8Hz), 128.64(m), 126.39(s), 125.33(d, J=12.0Hz), 124.35(d, J=10.9Hz), 123.80(s ), 121.87 (d, J=13.6Hz).31P NMR (162MHz, CDCl3): δ2.04.ESI-MS (m / z): [M-H]-calculated C26H18O3PS2, 473.660435;
[0074] Measured: 473.0417.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap