Amino acid dehydrogenase mutant and application thereof
An amino acid and dehydrogenase technology, applied in application, enzyme, genetic engineering and other directions, can solve the problem that amino acid dehydrogenase is not suitable for industrial production, and achieve the effects of improving enzyme specificity, reducing cost, and improving enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0029] According to a typical embodiment of the present invention, an amino acid dehydrogenase mutant is provided. The amino acid sequence of the amino acid dehydrogenase mutant is an amino acid sequence obtained by mutation of the amino acid sequence shown in SEQ ID NO: 1, and the mutation includes at least one of the following mutation sites: 64th, 94th, and 133rd , 137th, 148th, 168th, 173rd, 183rd, 191st, 207th, 229th, 248th, 255th and 282nd, and the 64th Lysine at position 94 is mutated to aspartate; aspartate at position 94 is mutated to alanine, glycine, valine, or serine; cysteine 133 is mutated to alanine or threonine acid; phenylalanine at position 137 is mutated to alanine; phenylalanine at position 148 is mutated to valine or alanine; asparagine at position 168 is mutated to aspartic acid; 173 Threonine at position 183 is mutated to serine, histidine, tryptophan, phenylalanine, or leucine; arginine at position 183 is mutated to phenylalanine, lysine, cysteine, v...
Embodiment 1
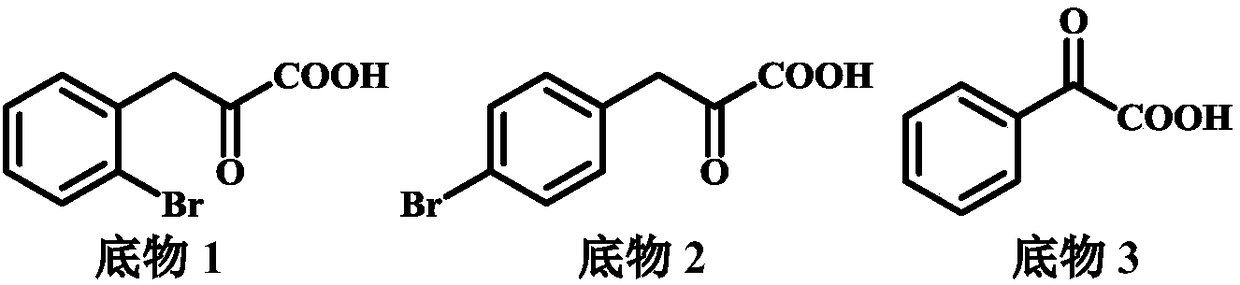
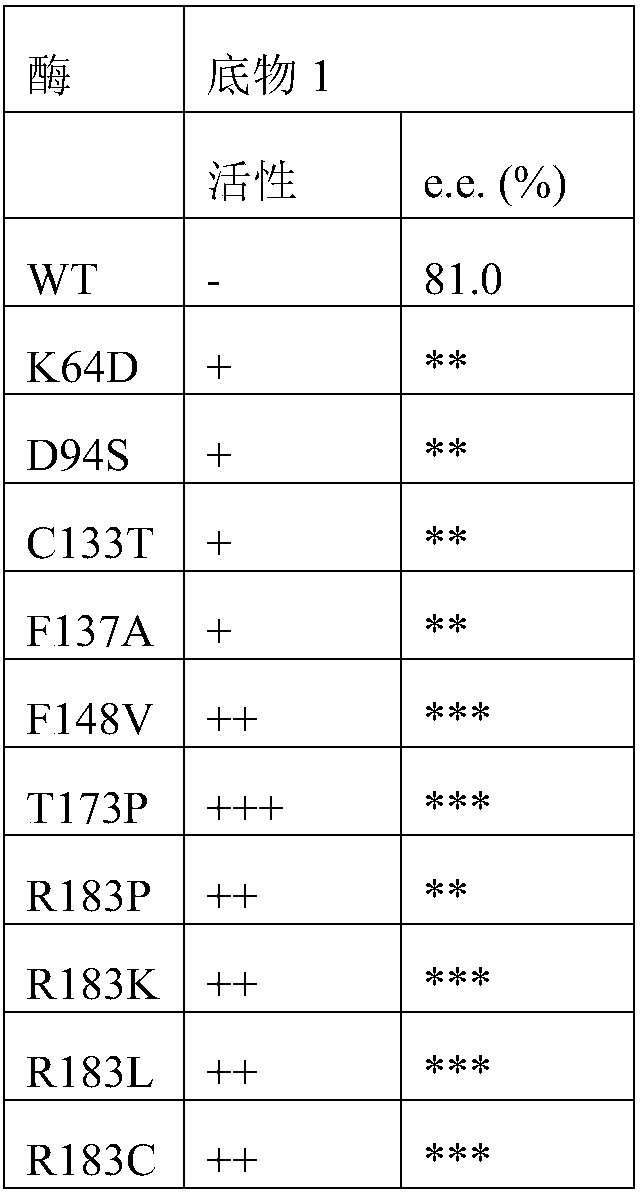
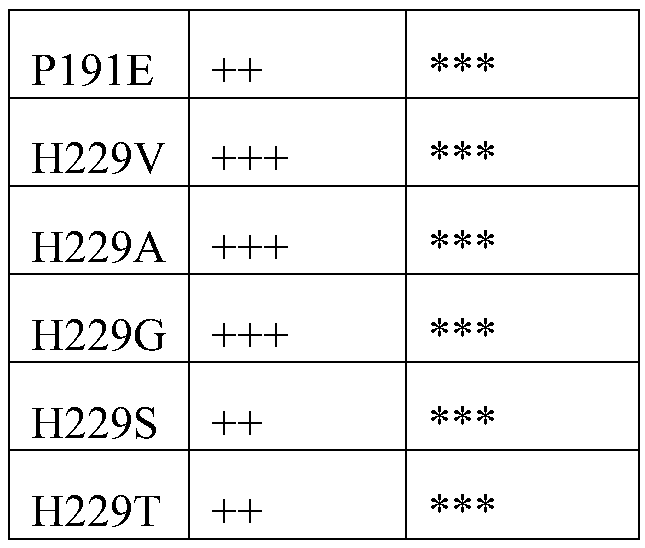
[0048] Add 20mg of substrate 1, 1mL reaction system, 22mg ammonium chloride, 30mg glucose, 4mg glucose dehydrogenase, NAD + 0.4mg, amino acid dehydrogenase 4mg, 0.1M Tris-HCl buffer. React at 30°C for 18 hours, add 900 μL (0.1N HCl:MeOH=1:1) to 100 μL system, centrifuge at 12000 rpm for 3 min, and take the supernatant for detection. Method for detecting ee value: Take 100 μL reaction system, add 100 μL ACN, 100 μL H 2 O, 100 μL 1M NaHCO 3 , centrifuge at 12000rpm for 3min, take out the supernatant, add 200μL of 5mg / mL Na-(2,4-dinitro-5-fluorophenyl)-L-alanamineamide, incubate at 50℃ for 1h, add 500μL of ACN and centrifuge, take the supernatant for liquid phase analysis.
[0049]
[0050]
[0051] Compared with the multiple of the mother parent, the activity is increased by 1-5 times, ++ by 5-10 times, +++ by 10-50 times, and ++++ by more than 50 times.
[0052] ee value between 80-90%*, ee value between 90-98%**, ee value greater than 98%***
Embodiment 2
[0054] Add 20mg of substrate 2, 1mL reaction system, 22mg ammonium chloride, 30mg glucose, 4mg glucose dehydrogenase, NAD + 0.4mg, amino acid dehydrogenase 4mg, 0.1M Tris-HCl buffer. React at 30°C for 18 hours, add 900 μL (0.1N HCl:MeOH=1:1) to 100 μL system, centrifuge at 12000 rpm for 3 min, and take the supernatant for detection. Method for detecting ee value: Take 100 μL reaction system, add 100 μL ACN, 100 μL H 2 O, 100 μL 1M NaHCO 3 , centrifuge at 12000rpm for 3min, take out the supernatant, add 200μL of 5mg / mL Na-(2,4-dinitro-5-fluorophenyl)-L-alanamineamide, incubate at 50℃ for 1h, add 500μL of ACN and centrifuge, take the supernatant for liquid phase analysis.
[0055]
[0056]
[0057] Compared with the multiple of the mother parent, the activity is increased by 1-5 times, ++ by 5-10 times, +++ by 10-50 times, and ++++ by more than 50 times.
[0058] ee value between 80-90%*, ee value between 90-98%**, ee value greater than 98%***
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com