Phthalide derivative as well as preparation method and application thereof
A technology of phthalides and derivatives, which is applied in the field of phthalide derivatives and their preparation, can solve problems such as not being able to meet the requirements for the treatment of stroke, and achieve good anti-platelet aggregation, stable chemical structure, and enhanced stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
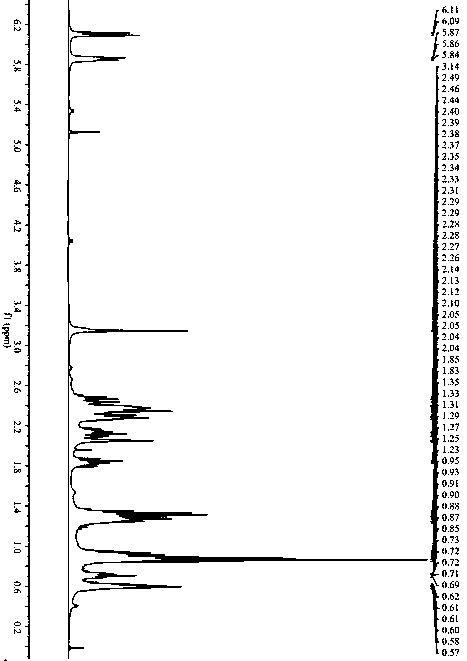
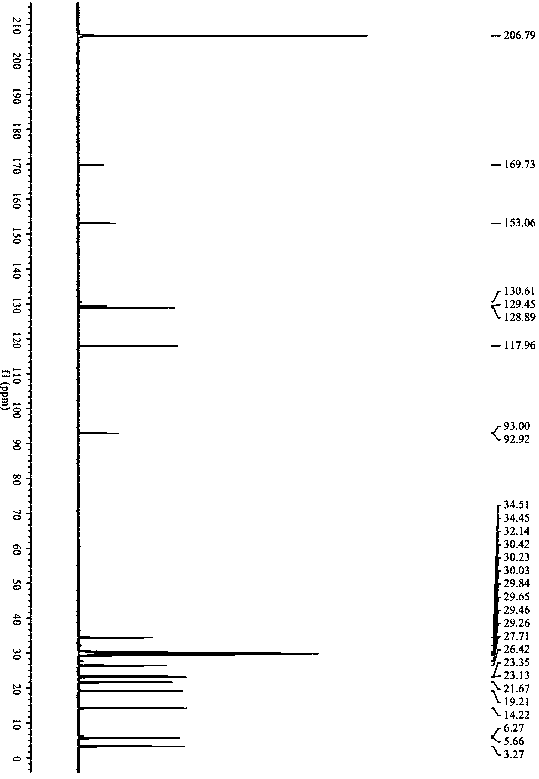
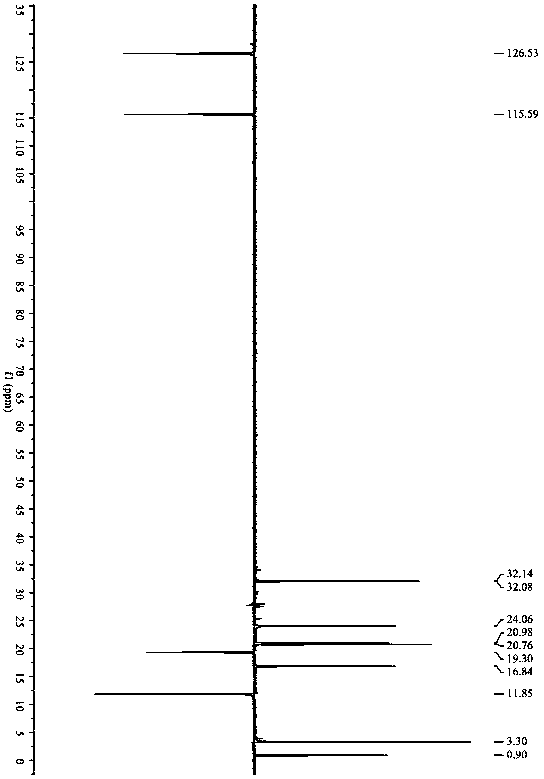
[0046] Example 1: N-cyclopropyl-3-n-butyl-3- S - Synthesis of hydroxy-ligustilactam.
[0047]Ligustilide (1.900 g, 0.010 mol) was dissolved in 50 mL of tetrahydrofuran, and cyclopropylamine (0.684 g, 0.012 mol) was dissolved in 20 mL of tetrahydrofuran. The temperature of the water bath was controlled at 25 ° C. Under mechanical stirring, the ring The tetrahydrofuran solution of propylamine was added dropwise to the tetrahydrofuran solution of ligustilide, stirred and reacted for 4 h, the tetrahydrofuran and excess cyclopropylamine solution were recovered by rotary evaporation under reduced pressure, and 200 mL of petroleum ether was added to the above concentrate, mixed evenly, and placed The synthetic product was crystallized and washed out overnight, and suction filtered to obtain 1.976 g of crude product, with a reaction yield of 80%. The crude product was recrystallized with a mixed solvent of petroleum ether and acetone (5:1, V / V) to obtain a crystalline product of 0.97 ...
Embodiment 2
[0051] Example 2: Synthesis of N-cyclopropyl-3-n-butyl-3-hydroxy-phthalide.
[0052] Ligustilide (1.880 g, 0.010 mol) was dissolved in 50 mL of tetrahydrofuran, and cyclopropylamine (0.684 g, 0.012 mol) was dissolved in 20 mL of tetrahydrofuran. Under mechanical stirring at room temperature, the tetrahydrofuran solution of cyclopropylamine was added dropwise to into the tetrahydrofuran solution of ligustilide, stirred and reacted for 6 hours, recovered tetrahydrofuran and excess cyclopropylamine solution by rotary evaporation under reduced pressure, added 200 mL of petroleum ether to the above concentrate, mixed well, and left overnight until the crystallization of the synthesized product was washed. out, and suction filtered to obtain 2.083 g of crude product, with a reaction yield of 85%. The crude product was recrystallized with a mixed solvent of petroleum ether and acetone (10:1, V / V), to obtain 1.666 g of crystalline product, with a crystalline yield of 80%. %. Using th...
Embodiment 3
[0055] Embodiment 3: Using angelica volatile oil to carry out compound N-cyclopropanyl-3-n-butyl-3-hydroxyl-ligustilactam and compound N-cyclopropyl-3-n-butyl-3-hydroxyl-phthalide Synthesis of a mixture of amides
[0056] The prepared Angelica volatile oil was pre-separated by column chromatography with petroleum ether and ethyl acetate (3:1, V / V) as the eluent, and the Angelica volatile oil (3.000 g ,) was dissolved in 60 mL of tetrahydrofuran, cyclopropylamine (0.684 g, 0.012 mol) was dissolved in 30 mL of tetrahydrofuran, under mechanical stirring, the tetrahydrofuran solution of cyclopropylamine was added dropwise to the above tetrahydrofuran solution of angelica volatile oil, and the reaction was stirred After 10 h, the tetrahydrofuran and excess cyclopropylamine solution were recovered by rotary evaporation under reduced pressure, and 200 mL of petroleum ether was added to the above concentrate, mixed evenly, and left overnight until the crystals of the synthesized produ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com