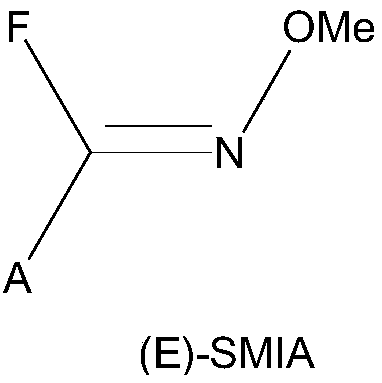
Method for converting furan ammonium salt cis-trans isomer
A technology of furan ammonium salt and cis-trans isomerization, which is applied in the field of furan ammonium salt cis-trans isomer conversion, can solve the problems of difficult ultraviolet protection, strong odor, and low conversion rate at one time, and achieves a green and environmentally friendly production process. Simple, production cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Choose a catalyst C (Fe 3 P), and then use the usual catalytic hydrogenation method to perform catalytic hydrogenation to obtain a catalyst O-I with hydride active points.
[0037] 420g furan ammonium salt trans by-product (E)-SMIA was dissolved in 1000ml dichloromethane, then added to a 2L four-necked reaction flask equipped with a stirrer, and 0.5g pre-hydrotreated with hydride active Point of catalyst O-I. The temperature in the reaction kettle was controlled at 0°C under stirring for 1 hour, and the reaction process was monitored by HPLC. The reaction solution obtained after the reaction was tested by HPLC, Z / E=99.5 / 0.5 (the ratio of the peak area of the Z formula to the E formula). The reaction liquid is filtered to recover the catalyst, and the existing known separation method is processed to obtain 398 g of furan ammonium salt product, with a product conversion rate of 99.5% and a product yield of 95%. The product quality inspection results obtained are as follo...
Embodiment 2
[0047] The process of converting furan ammonium salt trans by-product (E)-SMIA into furan ammonium salt product (Z)-SMIA is exactly the same as in Example 1. By changing the type and amount of the dilution solvent used, the ratio of the catalyst to the trans isomer, the reaction time, the reaction temperature, and other variables, Examples 2-15 were completed. The specific test result data is shown in Table A.
[0048] Table A Example 2-15 Experimental Data Statistics Table
[0049]
[0050]
Embodiment 16
[0052] Choose a catalyst C (Cu 3 P), and then use the usual catalytic hydrogenation method to carry out catalytic hydrogenation to obtain catalyst O-II with hydride active points.
[0053] 420kg of furan ammonium salt trans by-product (E)-SMIA was dissolved in 1000L of dichloromethane, then added to a 2000L enamel reactor equipped with a stirrer, and 0.5kg of pre-hydrotreated hydride active point was added The catalyst O-II. The temperature in the reactor was controlled at 0-3°C, and the reaction was stirred for 1 hour. The reaction liquid obtained after the reaction was tested by HPLC, Z / E=99.7 / 0.3. After the reaction is completed, the reaction mixture flows out of the reactor through the filter at the bottom of the reactor, and the catalyst remains in the reactor to continue the next batch of reaction. The conversion liquid excluding the reaction kettle is processed by an existing known separation method to obtain 408 kg of furan ammonium salt product, with a product conversi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com