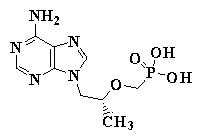
Tenofovir synthesizing method
A technology of tenofovir and its synthesis method, which is applied in the field of medicine and chemical industry, can solve the problems of high cost, strong corrosion, and three wastes, and achieve the effects of high risk, high selectivity, and reduced occurrence of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
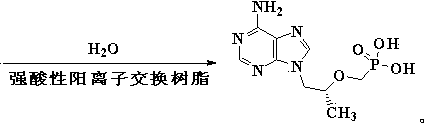
[0036] Example 1 Synthesis of Tenofovir
[0037] 57.9 g (0.30 mol) (R)-9-(2-hydroxypropyl) adenine was dissolved in 500 mL of DMF, and 165.6 g (alkali content 35%, 0.42 mol) freshly prepared solid base catalyst was added under good stirring K 2 CO 3 / Al 2 o 3 112.7 g (0.35 mol) diethyl p-toluenesulfonyloxymethylphosphonate was added dropwise below 40°C, the temperature was raised to 133-138°C after the drop, and the reaction was stirred for 12 hours. The solid base catalyst was recovered by filtration, and the DMF was recovered by distillation under reduced pressure. After cooling slightly, 600 ml of pure water and a strongly acidic cation exchange resin catalyst equivalent to 0.05 mole of acid were added. Stir and react at 105-110°C for 20 hours, filter and recover the strong acidic cation exchange resin catalyst, cool, filter, wash, and dry to obtain 78.3 g of crude tenofovir, with a yield of 90.9% and a content of 96.9%. The HPLC retention time and tenofovir The standa...
Embodiment 2
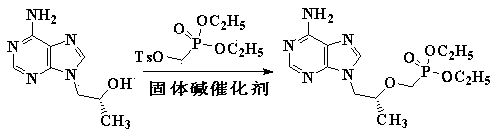
[0038] Example 2 Synthesis of Tenofovir
[0039] 57.9 g (0.30 mol) of (R)-9-(2-hydroxypropyl) adenine was dissolved in 330 mL of NMP, and 116.7 g (base content 18%, 0.375 mol) of freshly prepared solid base catalyst was added under good stirring KOH / Al 2 o 3 , Add 125.6 g (0.39 mol) diethyl p-toluenesulfonyloxymethylphosphonate dropwise below 40°C, raise the temperature to 110-115°C after dropping, and stir for 20 hours. The solid base catalyst is recovered by filtration, the NMP is recovered by distillation under reduced pressure, slightly cooled, and 400 milliliters of pure water and a strongly acidic cation exchange resin catalyst equivalent to 0.10 moles of acid are added. Stir and react at 102-107°C for 16 hours, filter and recover the strong acidic cation exchange resin catalyst, cool, filter, wash, and dry to obtain 77.7 g of crude tenofovir, with a yield of 90.2% and a content of 96.5%. The HPLC retention time and tenofovir The standard matches.
Embodiment 3
[0040] Example 3 Synthesis of Tenofovir
[0041] 57.9 g (0.30 mol) of (R)-9-(2-hydroxypropyl)adenine was dissolved in 380 mL of DMAc, and 62.6 g (base content 50%, 0.54 mol) of freshly prepared solid base catalyst was added under good stirring KF / Al 2 o 3 , Add 108.2 g (0.336 mol) diethyl p-toluenesulfonyloxymethylphosphonate dropwise below 40°C, raise the temperature to 98-103°C after dropping, and stir for 24 hours. The solid base catalyst was recovered by filtration, the DMAc was recovered by distillation under reduced pressure, cooled slightly, and 350 ml of pure water and a strongly acidic cation exchange resin catalyst equivalent to 0.03 moles of acid were added. Stir and react at 93-98°C for 28 hours, filter and recover the strong acidic cation exchange resin catalyst, cool, filter, wash, and dry to obtain 78.6 g of crude tenofovir, with a yield of 91.3% and a content of 97.3%. The standard matches.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com